| Citation: | Shicui Zhang, Mengmeng Yi. Amphioxus endostyle and origin of vertebrate thyroid[J]. Acta Oceanologica Sinica. |
The term thyroid gland, or thyroid for short, is originated from Greek thyreoeides, meaning shield-like/shield-shaped. It is interesting that the organ was initially known by ancient people through their observation of the thyroid-associated disease named goiter today. This can be illustrated by a review of the classical works home and abroad. In the Chinese classic book Shan Hai Jing (i.e., the Classic of Mountains and Rivers) written in about 700 BC, there are already the records of enlargement in the front of the neck, which was then called 瘿瘤Ying Liu (tumor of the neck) or 瘿囊Ying Nang (swelling of the neck). In another Chinese classic book Lü Shi Chun Qiu (i.e., Lü’s Commentaries of History) written in about 300 BC, there exists the description that “In areas with low salt and mineral contents, more people are found to have baldness and throat diseases”, which reveals a close relationship between the thyroid disease and geographical environment. Similarly, in the writings of Hippocrates (460-377 BC), he applied the term choiron, which was later used by the Paul of Aegina (625-590 BC), an ancient Greek medical encyclopaedist, to refer to goiter (Taylor, 1953; Toni, 2000; Konstantinidou and Konstantinidou, 2018). Still later, in the book De Usu Partium (On the usefulness of the parts of the human body) of Galen of Pergamum (129-200 AD), a Greek physician, he described the thyroid as “soft flesh in the neck”, and a ductless gland filtering the blood to produce “humor” moistening the larynx, providing the anatomical basis for the autonomic and vascular supply to the organ. It is noteworthy that it was Thomas Wharton (
All vertebrates are characteristic of a definitive thyroid gland. Anatomically, several cell types make up the thyroid gland, including follicular cells, endothelial cells, parafollicular cells (calcitonin-producing cells), and fibroblasts, lymphocytes, and adipocytes. Today we have had a comprehensive understanding of the morphology, structure, physiology, biochemistry and development of thyroid gland (Fagman and Nilsson, 2010, 2011; Stoupa et al., 2016; Eng and Lam, 2020). However, the study on its origin and evolution has only received due attention in recent decades, though it had long been an object of research in evolutionary biology (Müller, 1873; Hatschek, 1892).
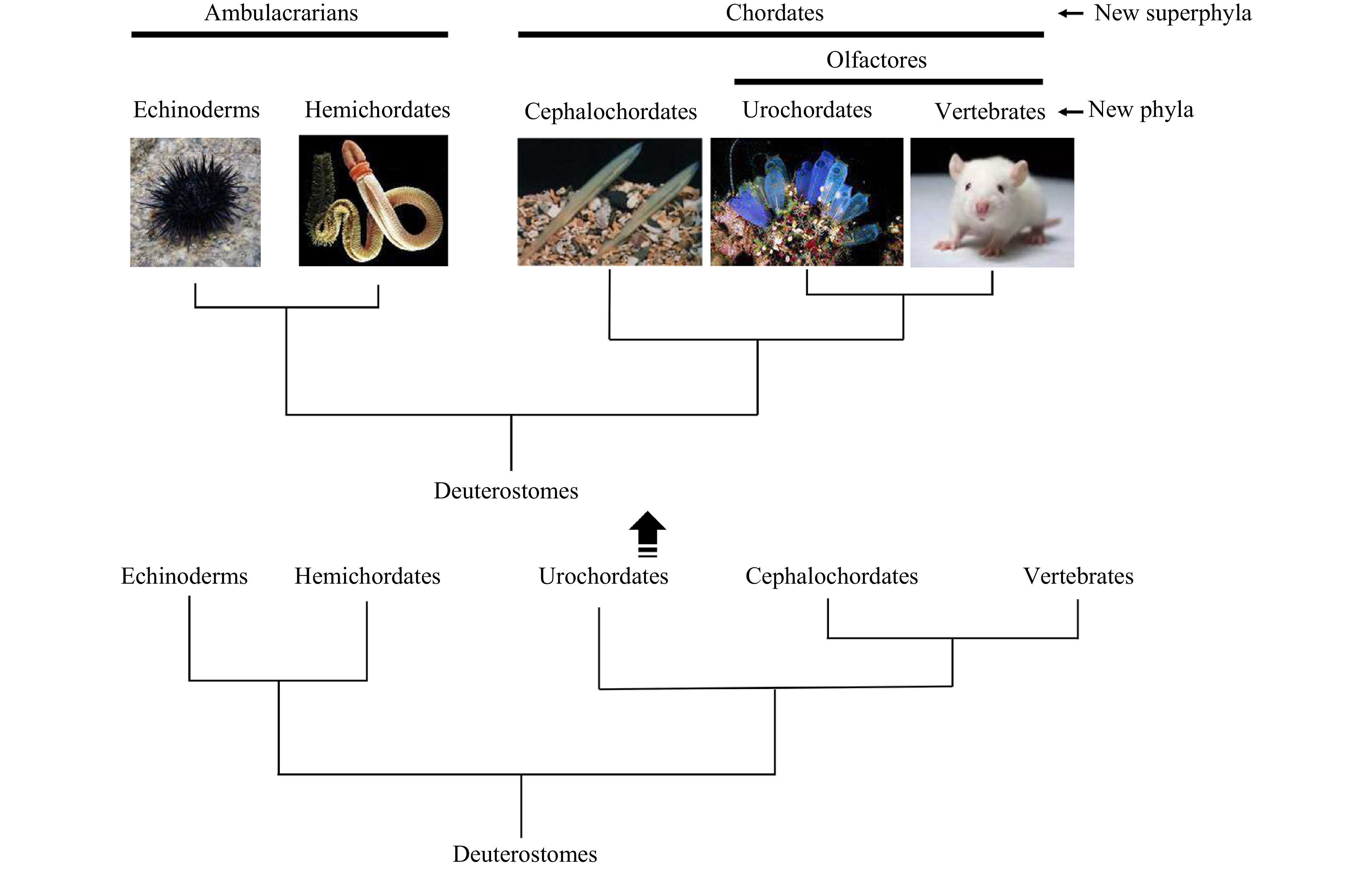
Traditionally, the superphylum Deuterostomia consists of the phyla Echinodermata, Hemichordata and Chordata. The Chordata characterized by presence of a notochord, a dorsal and hollow neural tube (nerve cord), myotomes and a postanal tail comprises three subphyla Urochordata (Tunicata), Cephalochordata and Vertebrata, that are thought to have originated from a common ancestor of the deuterostomes (Schaeffer, 1987; Cameron et al., 2000; Swalla and Smith, 2008; Nielsen, 2012; Satoh et al., 2014). Previously, a majority of biologists have favored an evolutionary scenario in which urochordates (ascidian) evolved first, then cephalochordates (amphioxus) and vertebrates (Fig. 1). However, recent studies of molecular biology and developmental genetics have unambiguously revealed that echinoderms and hemichordates form a clade, called the Ambulacraria which possesses similarities in coelomic systems and larvae (Metchnikoff, 1881), and that amphioxus, ascidian and vertebrates form another distinct clade, i.e., Chordata (Fig. 1) (Wada and Satoh, 1994; Cameron et al., 2000; Perseke et al., 2013). Moreover, phylogenetic analyses also reversed the positions of ascidian and amphioxus, placing ascidian as sister group (sometimes known as Olfactores, with similarities in extensive pharyngeal re-modification leading to the generation of new structures that are lacking in amphioxus) of the vertebrates and amphioxus basally in the chordates (Bourlat et al., 2006; Delsuc et al., 2006; Putnam et al., 2008). Hence, amphioxus is one of the best available stand-ins for the proximate invertebrate ancestor of vertebrates, and thus is an excellent model for the study of the origin and evolution of vertebrate traits. Here we will discuss the progress on the study of the evolutionary origin of vertebrate thyroid gland.

The thyroid gland is the anteriormost organ which develops from foregut endoderm. In mammals, the thyroid has a dual embryological origin: the foregut endoderm gives rise to the median anlage, while the paired ultimobranchial bodies (UB) deriving from the fourth pharyngeal pouches form the two lateral anlages. The development of thyroid gland has been studied extensively in mouse, and apparently the embryogenesis of the thyroid gland in humans follows the same pattern. The development of mouse thyroid gland starts at embryonic day 8.5 (E8.5) as an endodermal thickening in the floor of the primitive pharynx, which is located in the posterior portion of the mouth cavity. The cells of the endodermal thickening form the median thyroid anlage in the pharyngeal floor. At the molecular level, these cells are specified to a thyroid fate by co-expression of a set of transcription factors, including Nkx2-1 (formerly called TTF-1), FoxE (formerly called TTF-2), Pax8 (or Pax2 in Xenopus or Pax2/5/8 in zebrafish) and Hhex, that are also important for the functional differentiation of the gland in late development and postnatally (Lazzaro et al., 1991; Plachov et al., 1990; Zannini et al., 1997; Thomas et al., 1998; Fernández et al., 2015; Nilsson and Fagman, 2017). In addition, Nodal signaling (upstream of Pax2, Nkx2.1 and Hex) and Hh signaling are both required for specification of pharyngeal endoderm as well as development of the thyroid gland (Elsalini et al., 2003; Fagman et al., 2004; Moore-Scott and Manley, 2005; Porazzi et al., 2009; Bain et al., 2016). At E9.5, the median anlage evaginates from the floor of the pharynx to become a diverticulum which extends caudally. The thyroid diverticulum breaks its connection with the floor of the pharynx at E11.5, reaches its final position in front of the trachea at E12.5, and fuses with the UBs that bring the parafollicular cells to the thyroid at E13.0 (De Felice and Di Lauro, 2004; Trueba et al., 2005; Fagman and Nilsson, 2010). Only at this stage do the thyroid follicular cells begin differentiation and express thyroid-specific genes such as those encoding thyroglobulin (Tg), thyroid-stimulating hormone (also known as thyrotropin, thyrotropic hormone, TSH) receptor (TSHR), dual oxidase (Duox, an NADPH oxidase), thyroperoxidase (also known as thyroid peroxidase, TPO) and the sodium/iodide symporter (NIS) (Di Lauro and De Felice, 2001; Nilsson and Fagman, 2017) that are indispensable for synthesis of thyroid hormones.
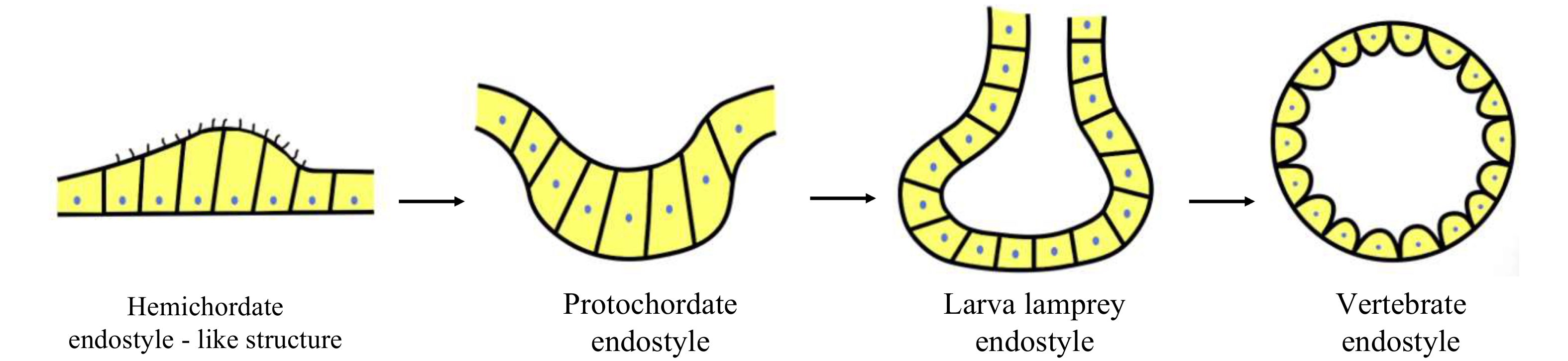
The thyroid gland of vertebrates was first proposed to have evolved from the endostyle (Fig. 2), an iodine-binding pharyngeal organ present in amphioxus (cephalochordate), ascidian (urochordate) and larval lampreys, by Müller (1873) who thought that an amphioxus-like endostyle in larval lampreys become converted to a thyroid gland in adult lampreys. In addition, Hatschek (1892) also implied that the club-shaped gland of amphioxus, in its entirety, is converted into the definitive endostyle, hinting at the clue that the club-shaped gland is the forerunner of the thyroid gland. The club-shaped gland was initially discovered in pre-metamorphic larvae by Schulze (1851). Since then, it has been regarded as a purely larval organ (Kowalevsky, 1867, 1877; Holland and Yu, 2002), and its functions and fate during the larva-to-juvenile metamorphosis have long been controversial. van Wijhe (1914) described one stage in which the club-shaped gland is reduced to a solid string of cells along the posterior endostyle band. Later studies also suggested that the cells of the club-shaped gland are incorporated into the post-metamorphic endostyle (Olsson, 1983; Gilmour, 1996; Yu et al., 2002). However, Fredriksson et al. (1984) thought that such incorporation was unlikely. Similarly, Holland et al. (2009) showed that the cells of the club-shape gland undergo massive apoptotic destruction during metamorphosis, and do not survive to participate in the genesis of the endostyle or any other post-larval structures, consistent with the view of early scholars (Willey, 1891; Orton, 1914; Franƶ, 1927; Goodrich, 1930). It is thus clear that the proposal regarding the homology between the amphioxus club-shaped gland and the vertebrate thyroid has not been widely accepted.
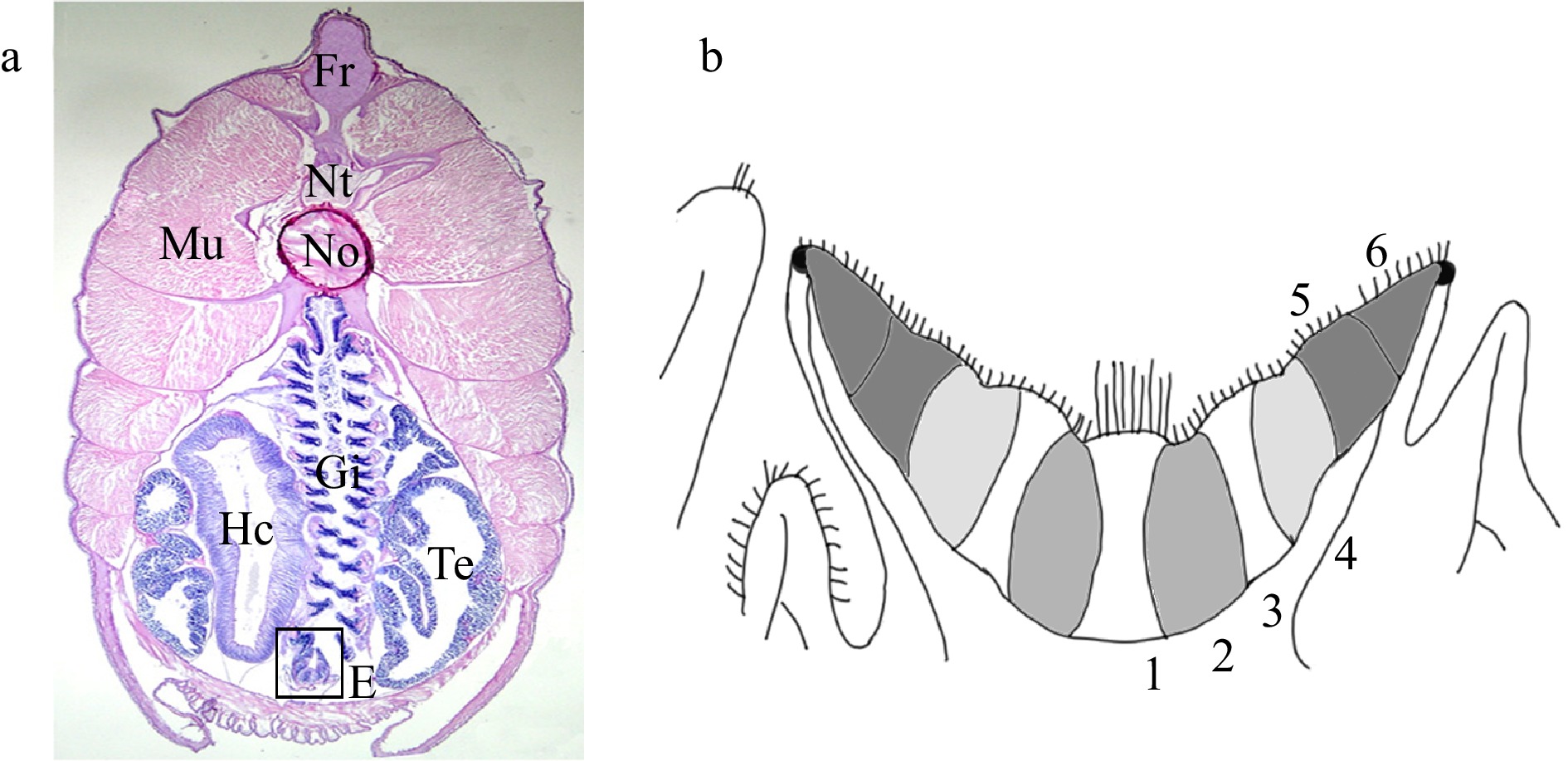
In amphioxus, the endostyle primordium originates from the anteroventral part of the pharyngeal endoderm, forming a groove on the ventral surface of the pharyngeal epithelium in larval stages, which comes to lie mainly on the right side as a result of rotation during metamorphosis. Therefore, the endostyle has an endodermal origin, and occurs in an anatomical position basically similar to that of the thyroid (Müller, 1873; Eales, 1997; Gorbman, 1955, 1997), indicating homology of the amphioxus endostyle to the vertebrate thyroid. This homology has gained additional supports from the biochemical, histochemical and developmental studies of amphioxus (Table 1). First, the amphioxus endostyle abounds in thyroxine T4 and triiodothyronine T3 (Wang et al., 2009). Second, the amphioxus endostyle includes several thyroid-related proteins involved in iodine metabolism (Colin et al., 2013), such as TPO, Duox and Tg, and can metabolize iodine to form iodothyronines (Tong et al., 1962; Monaco et al. 1981; Tsuneki et al., 1983; Fredriksson et al., 1984, 1985). Thirdly, the amphioxus endostyle expresses the thyroid-related transcription factors served as regulators of thyroid development in vertebrates (Fernändez et al., 2015; Onuma et al., 2021), such as Nkx2.1 (Venkatesh et al., 1999; Ogasawara, 2000; Benito-Gutiérrez et al., 2021), FoxE4 (Mazet, 2002; Hiruta et al., 2005; Yamagishi et al., 2022) and Pax2/5/8 (which is equally related to Pax2, Pax5 and Pax8 in tetrapods) (Kozmik et al., 1999; Hiruta et al., 2005). Finally, the Hh signaling pathway is essential for specification and patterning of the amphioxus pharynx containing gill slits and an endostyle, like that described for vertebrates, though the role of Nodal signaling in endostyle development remains controversial (Soukup et al., 2015; Wang et al., 2015; Ono et al., 2018).
The thyroid gland exerts its physiological roles mainly through the secretion of thyroid hormones (THs) T4 and T3. THs are produced by the follicular cells of the thyroid gland in all vertebrates and are transported through blood circulation. The major form of THs in the blood is the less potent T4, which is converted to T3, the more active hormone, within the target cells by deiodinases (St. Germain et al., 2009). A main target organ of THs is the liver, in which THs enter the cells, and bind to the nuclear receptors thyroid hormone receptors (TRs). The TH-TR complex formed acts directly on TH-sensitive genes to modulate their transcription (Menéndez-Hurtado et al., 1997; Harvey and Williams, 2004). For instance, T3 is able to induce a tissue specific expression of CCAAT/enhancer-binding proteins (C/EBP), C/EBPα and C/EBPβ, that play a critical role in energy metabolism in the liver (Matsuno et al., 1996; Crosson et al., 1997; Menéndez-Hurtado et al., 1997, 2000; Schrem et al., 2004; Pedersen et al., 2007). We together with others have shown that the amphioxus endostyle is rich in T4 and T3 (Fredriksson et al., 1985; Paris et al., 2008a, 2008b; Wang et al., 2009). We have also demonstrated that as in vertebrates, T4 and T3 and their derivative triiodothyroacetic acid (TRIAC) can significantly enhance the expression of the amphioxus C/EBPα/β gene (the archetype of vertebrate C/EBPα and C/EBPβ) in the hepatic caecum, an organ homologous to the liver, in a tissue-specific manner (Wang et al., 2009). Interestingly, iodopanoic acid (IOP), a deiodinase inhibitor, is able to inhibit the expression of C/EBPα/β gene in T4-treated animals, while it has little effect on animals treated with T3 or TRIAC, suggesting that the expression of C/EBPα/β gene is mainly induced by T3 generated through deiodination of T4 in amphioxus, as that in vertebrates. In addition, the recombinant peptide of amphioxus TR binds to both T3 and TRIAC, implicating that the expression of C/EBPα/β is mediated by interaction of T3/TRIAC with TR, conforming with the mode of action of THs in vertebrates. Other examples showing that the amphioxus THs function as those of vertebrates are the regulation of expression of insulin-like growth factors (IGFs) by T3. IGFs, small peptide growth factors, are primarily produced by the liver, and play an important role in regulating growth and metabolism. In vertebrates, THs stimulate the expression of IGF gene in the liver (Tsukada et al., 1998; Schmid et al., 2003; Leung et al., 2008), thereby modulating growth (Cao et al., 1989; Duan et al., 1993; Schmid et al., 2000; Li et al., 2010). Accordingly, our studies in vitro and in vivo both show that T3 is able to trigger the expression of IGF-like gene in the amphioxus hepatic caecum, in a dose-dependent manner (Wang and Zhang, 2011), suggesting the involvement of THs in the regulation of IGF gene expression in the amphioxus, as in vertebrates.
THs are also known to control metamorphosis in vertebrates such as amphibians and fish (Shi et al., 2001; Paris and Laudet, 2008; Buchholz et al., 2006). Numerous studies have identified THs and TRs as key components of gene networks mediating metamorphosis in vertebrates (Flamant et al., 2006). Upon TH binding, the TR initiates the modification of the transcription of target genes, eventually resulting in the morphological remodeling characteristic of metamorphosis (Tata, 2006). Similarly, TH and TR are also involved in amphioxus metamorphosis. It is found that metamorphosis in amphioxus is triggered by exogenous THs, while it is suppressed by inhibiting endogenous TH production (Paris et al., 2008b). Moreover, several amphioxus genes, including those encoding TR and deiodinase, that are involved in the TH signaling pathway, display an expression pattern correlated with metamorphosis, as in vertebrates (Paris et al., 2008b).
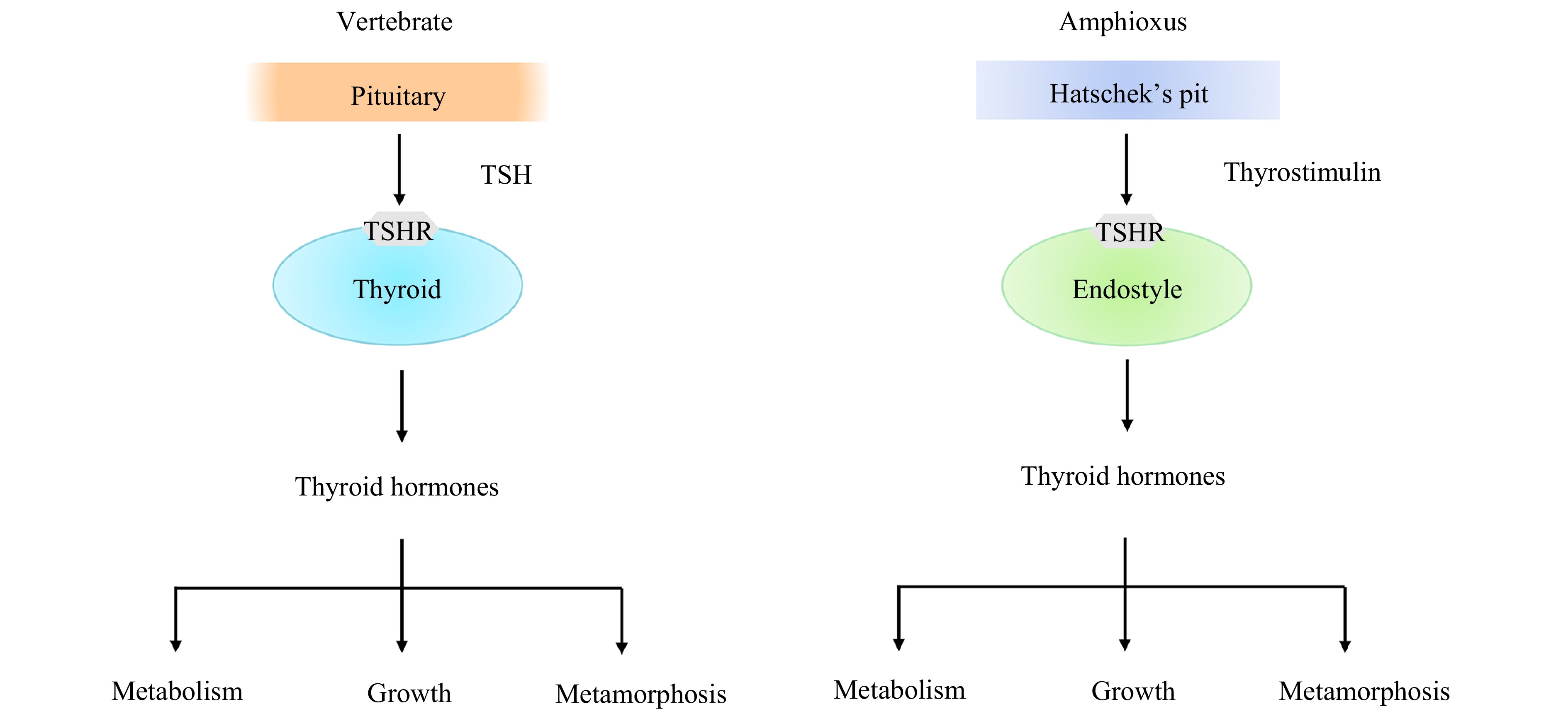
Production of THs by the thyroid gland is subjected to the regulation of thyroid-stimulating hormone, a pituitary hormone. TSH is a glycoprotein (Gp) consisting of two subunits, TSHα and TSHβ subunits. In general, TSH functions via binding to the TSH receptor (TSHR) on thyroid epithelial cells, eventually leading to secretion of THs. A previous study has shown that TSHR is present in amphioxus, but a genuine TSH is not (Paris et al., 2008a; Dong et al., 2013). We together with others demonstrate that amphioxus possesses a glycoprotein hormone (GpH), named thyrostimulin, as its sole GpH (Holland et al., 2008; Tando and Kubokawa, 2009a, 2009b; Dos Santos et al., 2009; Sower, 2015; Wang et al., 2018). The amphioxus thyrostimulin is composed of two distinct subunits known as GpA2 (α subunit) and GpB5 (β subunit). We also have found several lines of evidence showing that amphioxus thyrostimulin is a functional GpH which plays a role as TSH does in vertebrates (Wang et al., 2018). First, we show that amphioxus GpA2 and GpB5 as well as TSHR represent the archetypes of vertebrate TSHα, TSHβ, and TSHR, respectively. Second, both the genes coding for GpA2 and GpB5 are co-expressed in the Hatschek pit, an organ homologous to the vertebrate pituitary, in amphioxus. Thirdly, the recombinant amphioxus GpA2 and GpB5, resembling zebrafish TSHα and TSHβ, are capable of interacting with both amphioxus and zebrafish TSHR, and importantly, the tethered amphioxus thyrostimulin is capable of triggering both protein kinase A and protein kinase C pathways in the cells expressing amphioxus TSHR. Finally, the recombinant amphioxus thyrostimulin is able to induce the generation of T4. Altogether, our data suggest that thyrostimulin is able to interact with TSHR in amphioxus, thereby regulating TH production in a fashion similar to that of vertebrate TSH/TSHR system (Fig. 3).
The acquisition of the thyroid was regarded as one of seminal events in chordate evolution which led to the divergence and regulation of many physiological functions such as development, growth and metabolism, and hence its origin and evolution have received attention a long time ago. Exactly when and how did the thyroid gland evolve? Due to the sharing of many topological, developmental and physiological characteristics including endodermal origin, accumulation of iodide and peroxidase activity, and gene expression profiles, the thyroid gland of vertebrates is considered to have evolved from the endostyle, a ventral midline organ of the pharynx of the protochordates (urochordates and cephalochordates) and larval lampreys. The endodermal origin and iodinating capacity documented for the protochordate endostyle support its role as a thyroid forerunner in invertebrates. The expression of orthologous genes involved in the development and function of vertebrate thyroid in the protochordate endostyle provides a further support to this role. Intriguingly, the epithelial cells in the endostyle capable of iodination are located in zones on both dorsolateral sides of the endostylar wall, and do not show any follicular structure (Fredriksson et al., 1984, 1985, 1988), which contrasts to the thyroid follicles consisting of epithelial thyrocytes responsible for hormone production. Probably, the conversion of larval endostyle in lampreys to a thyroid gland in adult lampreys during metamorphosis represents a transitional stage in thyroid evolution towards a follicular endocrine gland (Marine, 1913; Wright and Youson, 1976, 1980; Kluge et al., 2005).
It is notable that the hypobranchial ridge, a row of multiciliated cells running along the ventral midline of the digestive pharynx in the hemichordates, has been proposed as a possible precursor to the protochordate endostyle because of its anatomical position, histological features and gene expression profiles (Welsch and Storch, 1970; Ruppert, 1997; Takacs et al., 2002; Satoh et al., 2014; Andrade Lόpez et al., 2023). Importantly, the endostyle cells of hemichordates bind iodine, though iodine binding occurs all throughout the pharynx (Ruppert, 2005). From a phylogenetic viewpoint, we propose that the hemichordate endostyle is the most primitive forerunner of the vertebrate thyroid, and the evolution of the vertebrate thyroid involves the transformation of non-follicular endostyle typical of the endostyle of the protochordates (amphioxus and ascidian) and larval lampreys to follicular endocrine organ characteristic of the thyroid of adult lampreys and jawed vertebrates (Fig. 4). However, a couple of questions remain to be clarified regarding the origin and evolution of the vertebrate thyroid. First of all, it is evident that only certain cells of the protochordate endostyle are the antecedents of the vertebrate thyroid follicle cells (Barrington, 1957, 1958, 1965; Barrington and Sage, 1972; Olsson, 1963, 1969), but it is still open how the distinct cell types are recruited from neighboring regions of the endostyle to form the endocrine thyroid of vertebrates. The broad pharynx in hemichordates binds iodine, and expresses the transcription factors such as NK2.1 and FoxE involved in the regulation of thyroid development, but if the pharyngeal cells can synthesize THs remains unknown; and if so, which cells correspond to the TH-producing cells still needs study. In vertebrates, TH production by the thyroid is mediated by the hypothalamic-pituitary-thyroid (HPT) axis, i.e., the hypothalamus produces TSH-releasing hormone (TRH), which stimulates the synthesis and secretion of TSH in the pituitary, and TSH in turn stimulates TH generation in the thyroid. Nevertheless, it now remains unknown if HPT axis emerges in the protochordates. The study of the questions above will greatly deepen and richen our understanding of the origin and evolution of the thyroid gland in vertebrates.