Dietary exposure to sulfamethazine, nanoplastics and their binary mixture disrupts the spermatogenesis of marine medaka (Oryzias melastigma)
-
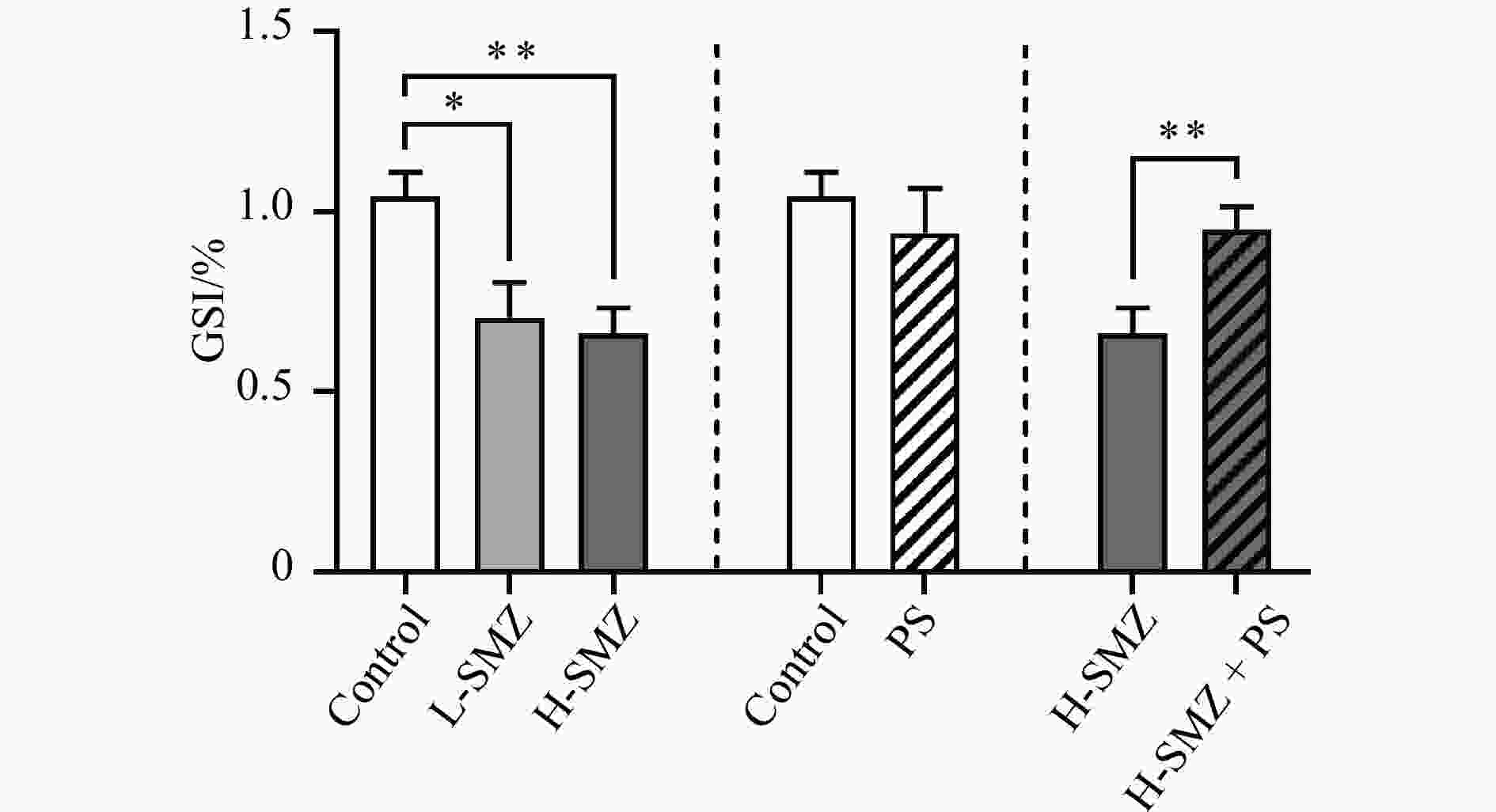
Abstract: In the coastal environment, the co-occurrence of antibiotic and nanoplastic pollution is common. Investigating their individual and combined toxicity to marine organisms is of great necessity. In the present study, the reproductive toxicity of sulfamethazine (SMZ) and nanoplastics (polystyrene, PS) via the dietary route on the spermatogenesis of marine medaka (Oryzias melastigma) was examined. After 30 d of dietary exposure, SMZ alone decreased the gonadosomatic index (GSI) value (~35%) and the proportion of undifferentiated type A spermatogonia (Aund) (~40%), probably by disrupting the testicular sex hormone production, the spermatogenesis-related growth factor network and the balance of apoptosis. Individual exposure to PS did not affect the GSI value or the proportions of germ cells at different developmental stages, but dysregulated the expression of several spermatogenesis-related genes. Interestingly, the presence of PS alleviated the decreased GSI value caused by SMZ. This alleviation effect was achieved by enhancing the spermatogonia differentiation instead of reversing the suppressed self-renewal of Aund, suggesting that the mixture of PS and SMZ could cause reproductive effects in a different way. These findings expand our knowledge of threats of ubiquitous antibiotic and nanoplastic pollution to fish reproduction and population.
-
Key words:
- nanoplastics /
- antibiotics /
- spermatogenesis /
- combined toxicity /
- Oryzias melastigma
-
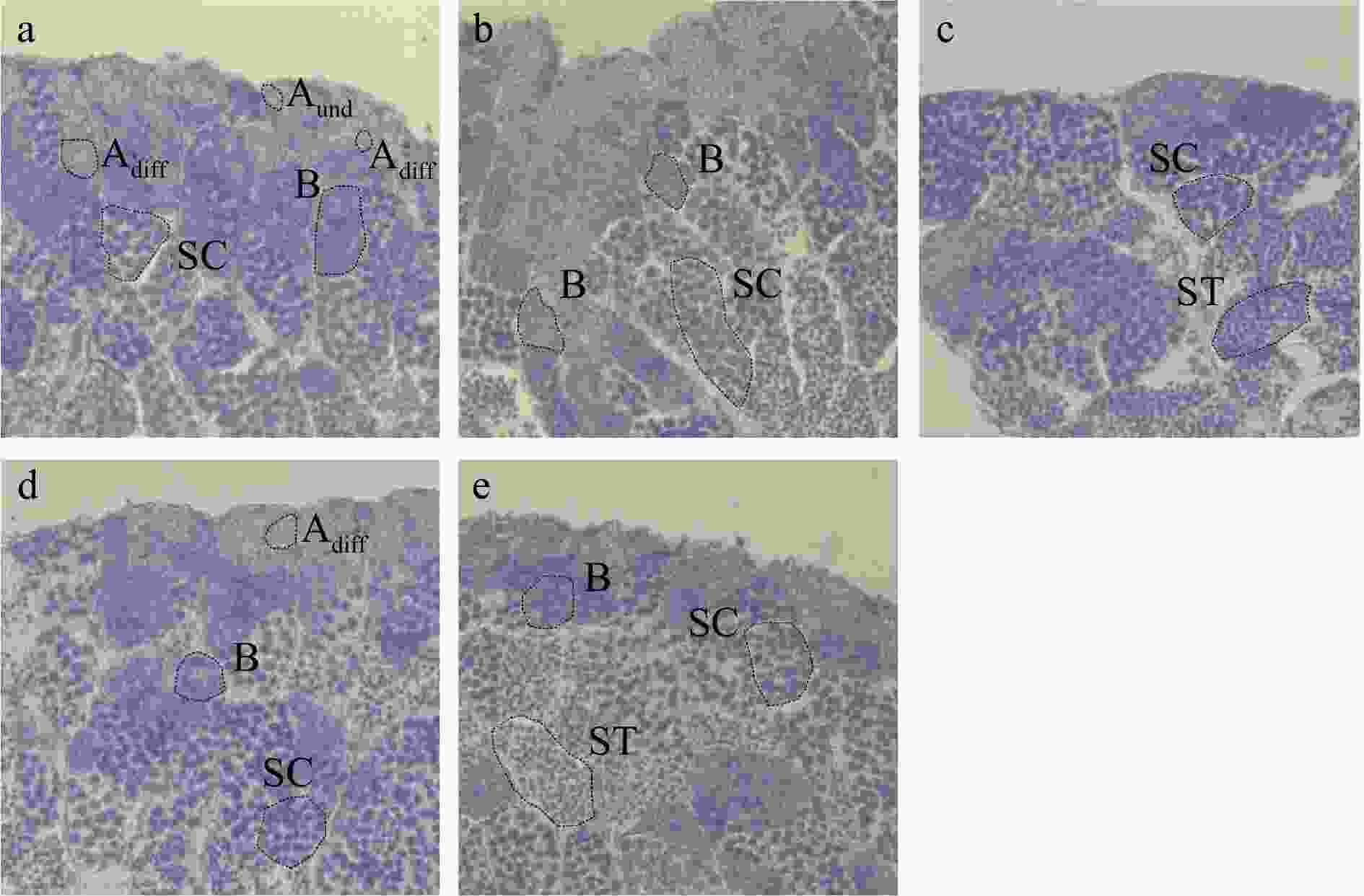
Figure 2. Paraffin sections of testis from the Control (a), L-SMZ (b), H-SMZ (c), PS (d) and H-SMZ + PS (e) groups. No significant effects of PS, SMZ or their binary mixture on the morphological structure of testis were observed. Aund, undifferentiated type A spermatogonia; Adiff, differentiated type A spermatogonia; B, type B spermatogonia; SC, spermatocyte; ST, spermatids.
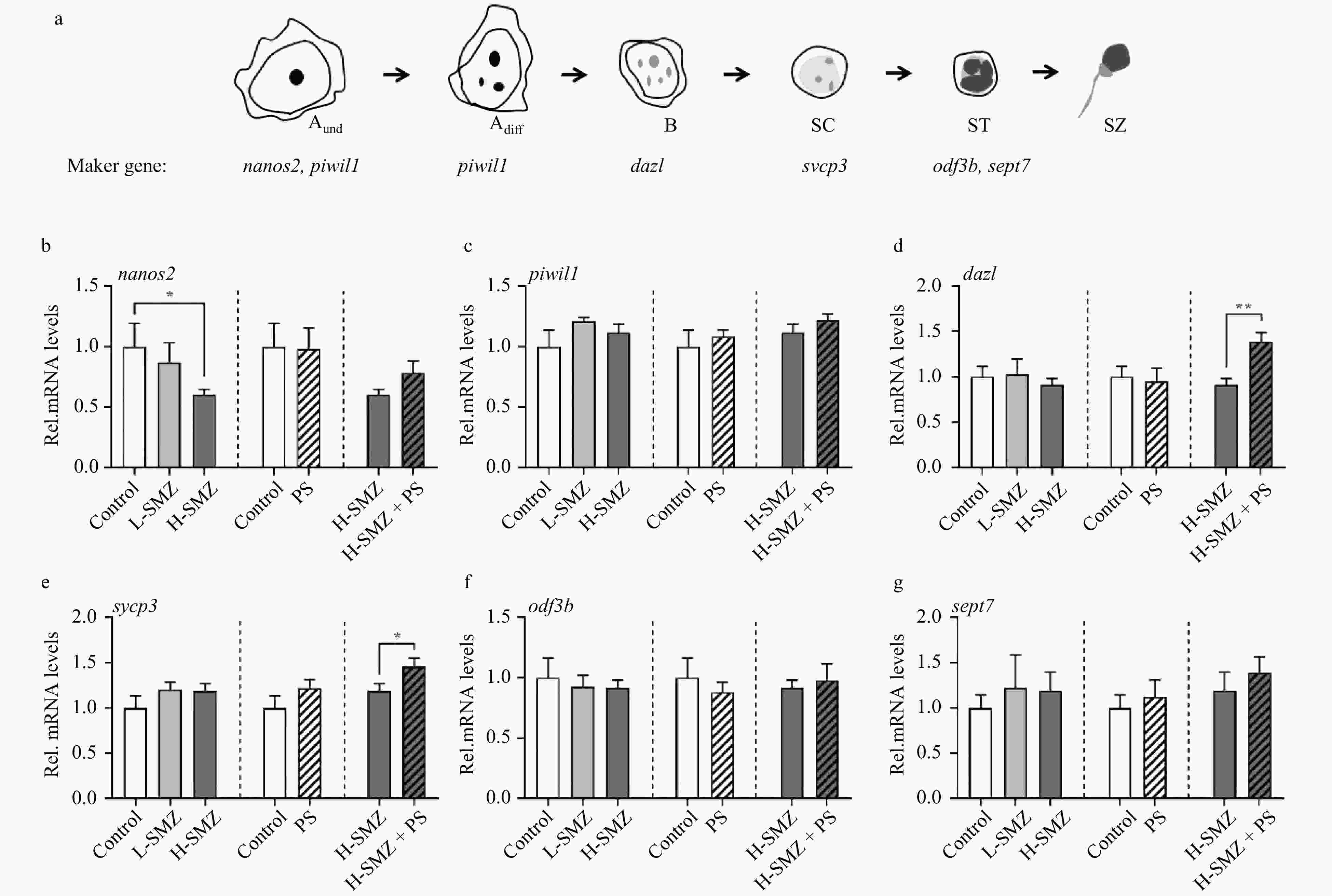
Figure 3. Relative expression of germ cell marker genes in the sulfamethazine (SMZ) and nano polystyrene (PS) exposed male O. melastigma. Representation of spermatogenesis from type A undifferentiated spermatogonia to spermatozoa was modified from Schulz et al., (2010) (a). The transcriptional expression levels of nanos2 (b), piwil1 (c), dazl (d), sycp3 (e), odf3b (f) and sept7 (g) were measured. Asterisks indicated significant differences (*0.01 < p < 0.05, ** p ≤ 0.01, n ≥ 5). Aund, undifferentiated type A spermatogonia; Adiff, differentiated type A spermatogonia; B, type B spermatogonia; SC, spermatocyte; ST, spermatids; SZ, spermatozoa.
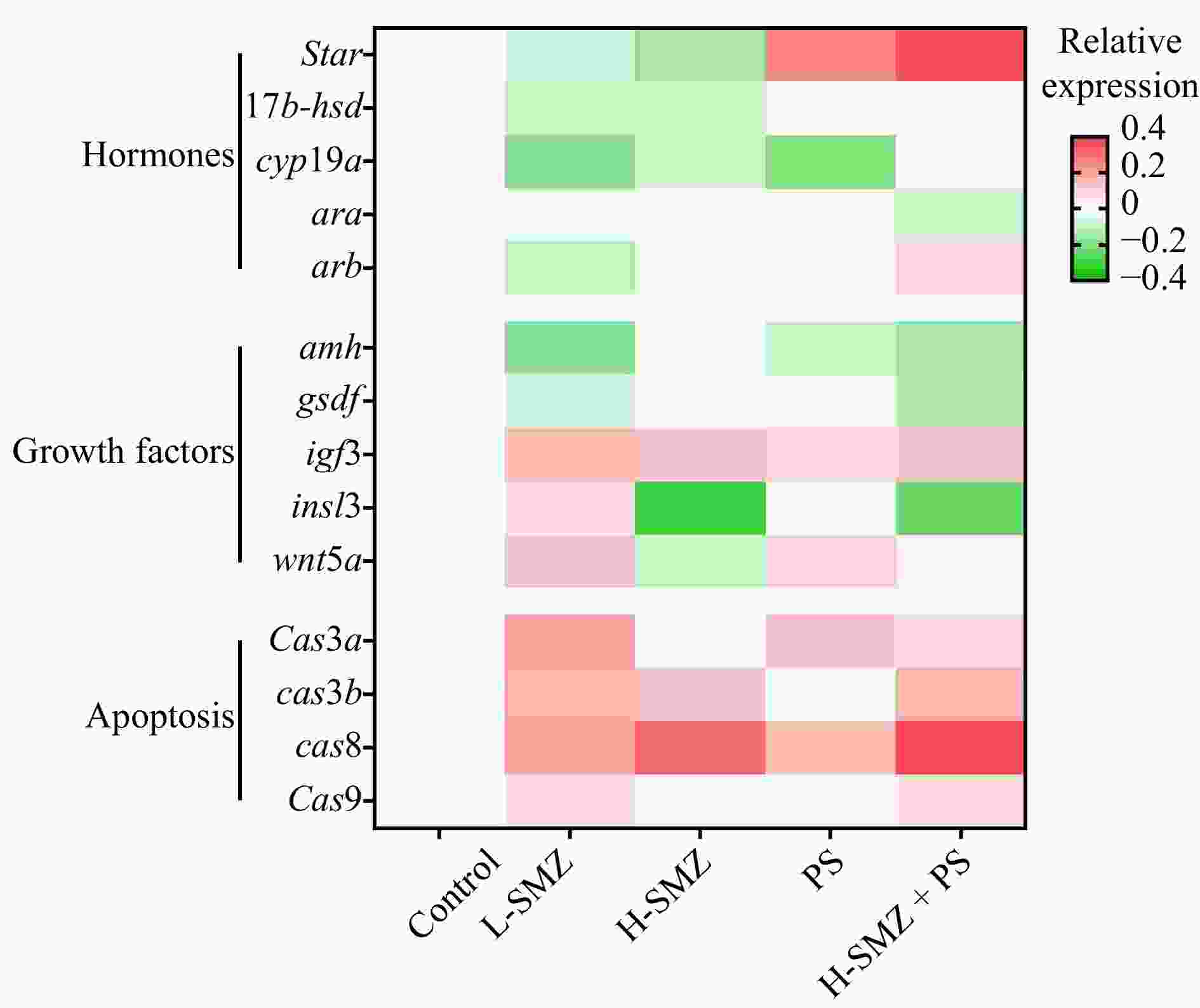
Figure 4. Relative expression of spermatogenesis related genes in the sulfamethazine (SMZ) and nano polystyrene (PS) exposed male O. melastigma. The values of four treatment groups were normalized to the control which was set to 0. Color bar from red to green represented the fold change from increasing to decreasing (n ≥ 5).
-
Abdolahpur Monikh F, Baun A, Hartmann N B, et al. 2023. Exposure protocol for ecotoxicity testing of microplastics and nanoplastics. Nature Protocols, 18(11): 3534–3564, doi: 10.1038/s41596-023-00886-9 Allen D, Allen S, Abbasi S, et al. 2022. Microplastics and nanoplastics in the marine-atmosphere environment. Nature Reviews Earth & Environment, 3(6): 393–405 Almeida C, Correia S, Rocha E, et al. 2013. Caspase signalling pathways in human spermatogenesis. Journal of Assisted Reproduction and Genetics, 30(4): 487–495, doi: 10.1007/s10815-013-9938-8 Andrady A L. 2011. Microplastics in the marine environment. Marine Pollution Bulletin, 62(8): 1596–1605, doi: 10.1016/j.marpolbul.2011.05.030 Avio C G, Gorbi S, Milan M, et al. 2015. Pollutants bioavailability and toxicological risk from microplastics to marine mussels. Environmental Pollution, 198: 211–222, doi: 10.1016/j.envpol.2014.12.021 Bu Qingwei, Wang Bin, Huang Jun, et al. 2013. Pharmaceuticals and personal care products in the aquatic environment in China: a review. Journal of Hazardous Materials, 262: 189–211, doi: 10.1016/j.jhazmat.2013.08.040 Crespo D, Assis L H C, Furmanek T, et al. 2016. Expression profiling identifies Sertoli and Leydig cell genes as Fsh targets in adult zebrafish testis. Molecular and Cellular Endocrinology, 437: 237–251, doi: 10.1016/j.mce.2016.08.033 Crespo D, Lemos M S, Zhang Yuting, et al. 2020. PGE2 inhibits spermatogonia differentiation in zebrafish: interaction with Fsh and an androgen. Journal of Endocrinology, 244(1): 163–175, doi: 10.1530/JOE-19-0309 De Liguoro M, Fioretto B, Poltronieri C, et al. 2009. The toxicity of sulfamethazine to Daphnia magna and its additivity to other veterinary sulfonamides and trimethoprim. Chemosphere, 75(11): 1519–1524, doi: 10.1016/j.chemosphere.2009.02.002 Gao Xiang, Ding Guanghui, Li Xishan, et al. 2018. Comparison of toxicity effects of fuel oil treated by different dispersants on marine medaka (Oryzias melastigma) embryo. Acta Oceanologica Sinica, 37(11): 123–132, doi: 10.1007/s13131-018-1255-8 Geens T, Aerts D, Berthot C, et al. 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food and Chemical Toxicology, 50(10): 3725–3740, doi: 10.1016/j.fct.2012.07.059 Guo Xuan, Liu Yong, Wang Jianlong. 2019. Sorption of sulfamethazine onto different types of microplastics: a combined experimental and molecular dynamics simulation study. Marine Pollution Bulletin, 145: 547–554, doi: 10.1016/j.marpolbul.2019.06.063 Hikim A P S, Swerdloff R S. 1999. Hormonal and genetic control of germ cell apoptosis in the testis. Reviews of Reproduction, 4(1): 38–47, doi: 10.1530/ror.0.0040038 Ji Xiuling, Shen Qunhui, Liu Fang, et al. 2012. Antibiotic resistance gene abundances associated with antibiotics and heavy metals in animal manures and agricultural soils adjacent to feedlots in Shanghai; China. Journal of Hazardous Materials, 235–236: 178–185 Jiang Lei, Hu Xialin, Yin Daqiang, et al. 2011. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere, 82(6): 822–828, doi: 10.1016/j.chemosphere.2010.11.028 Kim H S, Lee B Y, Han J, et al. 2018. The genome of the marine medaka Oryzias melastigma. Molecular Ecology Resources, 18(3): 656–665, doi: 10.1111/1755-0998.12769 Lalumera G M, Calamari D, Galli P, et al. 2004. Preliminary investigation on the environmental occurrence and effects of antibiotics used in aquaculture in Italy. Chemosphere, 54(5): 661–668, doi: 10.1016/j.chemosphere.2003.08.001 Li Bowen, Liang Weiwenhui, Liu Quanxing, et al. 2021. Fish ingest microplastics unintentionally. Environmental Science & Technology, 55(15): 10471–10479 Li Jia, Zhang Kaina, Zhang Hua. 2018. Adsorption of antibiotics on microplastics. Environmental Pollution, 237: 460–467, doi: 10.1016/j.envpol.2018.02.050 Limbu S M, Zhou Li, Sun Shengxiang, et al. 2018. Chronic exposure to low environmental concentrations and legal aquaculture doses of antibiotics cause systemic adverse effects in Nile tilapia and provoke differential human health risk. Environment International, 115: 205–219, doi: 10.1016/j.envint.2018.03.034 Manna P R, Stetson C L, Slominski A T, et al. 2016. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine, 51(1): 7–21, doi: 10.1007/s12020-015-0715-6 Materić D, Kjær H A, Vallelonga P, et al. 2022. Nanoplastics measurements in Northern and Southern polar ice. Environmental Research, 208: 112741, doi: 10.1016/j.envres.2022.112741 Ming Junchao, Fu Zhengyi, Ma Zhenhua, et al. 2020. The effect of sulfamonomethoxine treatment on the gut microbiota of Nile tilapia (Oreochromis niloticus). MicrobiologyOpen, 9(11): e1116, doi: 10.1002/mbo3.1116 Miura T, Miura C, Ohta T, et al. 1999. Estradiol-17β stimulates the renewal of spermatogonial stem cells in males. Biochemical and Biophysical Research Communications, 264(1): 230–234, doi: 10.1006/bbrc.1999.1494 Nóbrega R H, Morais R D V D S, Crespo D, et al. 2015. Fsh stimulates spermatogonial proliferation and differentiation in zebrafish via Igf3. Endocrinology, 156(10): 3804–3817, doi: 10.1210/en.2015-1157 Plottel C S, Blaser M J. 2011. Microbiome and malignancy. Cell Host & Microbe, 10(4): 324–335 Qi Xinyu, Yun Chuyu, Pang Yanli, et al. 2021. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes, 13(1): 1894070, doi: 10.1080/19490976.2021.1894070 Rist S, Baun A, Hartmann N B. 2017. Ingestion of micro-and nanoplastics in Daphnia magna–Quantification of body burdens and assessment of feeding rates and reproduction. Environmental Pollution, 228: 398–407, doi: 10.1016/j.envpol.2017.05.048 Schmittgen T D, Livak K J. 2008. Analyzing real-time PCR data by the comparative CT method. Nature Protocols, 3(6): 1101–1108, doi: 10.1038/nprot.2008.73 Schulz R W, de França L R, Lareyre J J, et al. 2010. Spermatogenesis in fish. General and Comparative Endocrinology, 165(3): 390–411, doi: 10.1016/j.ygcen.2009.02.013 Segner H. 2011. Chapter 86-Reproductive and developmental toxicity in fishes. In: Gupta R C, ed. Reproductive and Developmental Toxicology. San Diego: Academic Press, 1145–1166 Song M, Gutzeit H O. 2003. Effect of 17‐α‐ethynylestradiol on germ cell proliferation in organ and primary culture of medaka (Oryzias latipes) testis. Development, Growth & Differentiation, 45(4): 327–337 Trevisan R, Voy C, Chen Shuxin, et al. 2019. Nanoplastics decrease the toxicity of a complex PAH mixture but impair mitochondrial energy production in developing zebrafish. Environmental Science & Technology, 53(14): 8405–8415 Varshney S, Gora A H, Kiron V, et al. 2023. Polystyrene nanoplastics enhance the toxicological effects of DDE in zebrafish (Danio rerio) larvae. Science of the Total Environment, 859: 160457, doi: 10.1016/j.scitotenv.2022.160457 Wang Wenxiong. 2013. Dietary toxicity of metals in aquatic animals: recent studies and perspectives. Chinese Science Bulletin, 58(2): 203–213, doi: 10.1007/s11434-012-5413-7 Wang Xuedong, Ma Yan, Liu Jinfeng, et al. 2017. Reproductive toxicity of β-diketone antibiotic mixtures to zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 141: 160–170, doi: 10.1016/j.ecoenv.2017.02.042 Wang Jundong, Tan Zhi, Peng Jinping, et al. 2016. The behaviors of microplastics in the marine environment. Marine Environmental Research, 113: 7–17, doi: 10.1016/j.marenvres.2015.10.014 Xiang Keyu, He Zhiyu, Fu Jianxin, et al. 2022. Microplastics exposure as an emerging threat to ancient lineage: A contaminant of concern for abnormal bending of amphioxus via neurotoxicity. Journal of Hazardous Materials, 438: 129454, doi: 10.1016/j.jhazmat.2022.129454 Xiao Kun, Song Lili, Li Yishuai, et al. 2023. Dietary intake of microplastics impairs digestive performance, induces hepatic dysfunction, and shortens lifespan in the annual fish Nothobranchius guentheri. Biogerontology, 24(2): 207–223, doi: 10.1007/s10522-022-10007-w Xie Lingtian, Funk D H, Buchwalter D B. 2010. Trophic transfer of Cd from natural periphyton to the grazing mayfly Centroptilum triangulifer in a life cycle test. Environmental Pollution, 158(1): 272–277, doi: 10.1016/j.envpol.2009.07.010 Yan Zhengyu, Yang Qiulian, Jiang Weili, et al. 2018. Integrated toxic evaluation of sulfamethazine on zebrafish: including two lifespan stages (embryo-larval and adult) and three exposure periods (exposure, post-exposure and re-exposure). Chemosphere, 195: 784–792, doi: 10.1016/j.chemosphere.2017.12.119 Zhang Yuting, Chen Mengyun, He Shuiqing, et al. 2021a. Microplastics decrease the toxicity of triphenyl phosphate (TPhP) in the marine medaka (Oryzias melastigma) larvae. Science of The Total Environment, 763: 143040, doi: 10.1016/j.scitotenv.2020.143040 Zhang Yuting, Chen Hongxing, He Shuiqing, et al. 2021b. Subchronic toxicity of dietary sulfamethazine and nanoplastics in marine medaka (Oryzias melastigma): Insights from the gut microbiota and intestinal oxidative status. Ecotoxicology and Environmental Safety, 226: 112820, doi: 10.1016/j.ecoenv.2021.112820 Zhao Songhe, Wang Xinhong, Li Yongyu, et al. 2016. Bioconcentration, metabolism, and biomarker responses in marine medaka (Oryzias melastigma) exposed to sulfamethazine. Aquatic Toxicology, 181: 29–36, doi: 10.1016/j.aquatox.2016.10.026 Zheng RongHui, Fang Chao, Hong Fukun, et al. 2024. An innovative classification system for ranking the biological effects of marine aromatic hydrocarbons based on fish embryotoxicity. Acta Oceanologica Sinica, 43: 1–10 Zhou Li, Limbu S M, Qiao Fang, et al. 2018. Influence of long-term feeding antibiotics on the gut health of zebrafish. Zebrafish, 15(4): 340–348, doi: 10.1089/zeb.2017.1526 -
 Zhang Yuting_Supplementary information Zhang Yuting.pdf
Zhang Yuting_Supplementary information Zhang Yuting.pdf
-




 下载:
下载: