Geochemical speciation and spatial distributions of phosphorus in surface sediments from the basin of the Marcus-Wake seamounts in the western Pacific Ocean
-
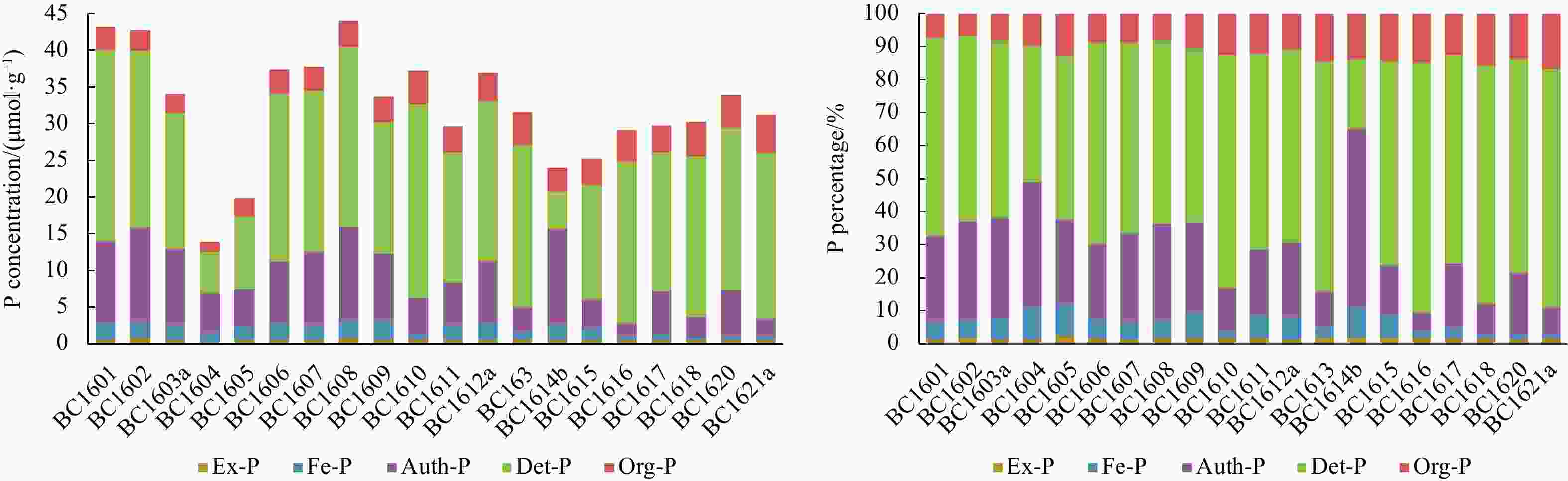
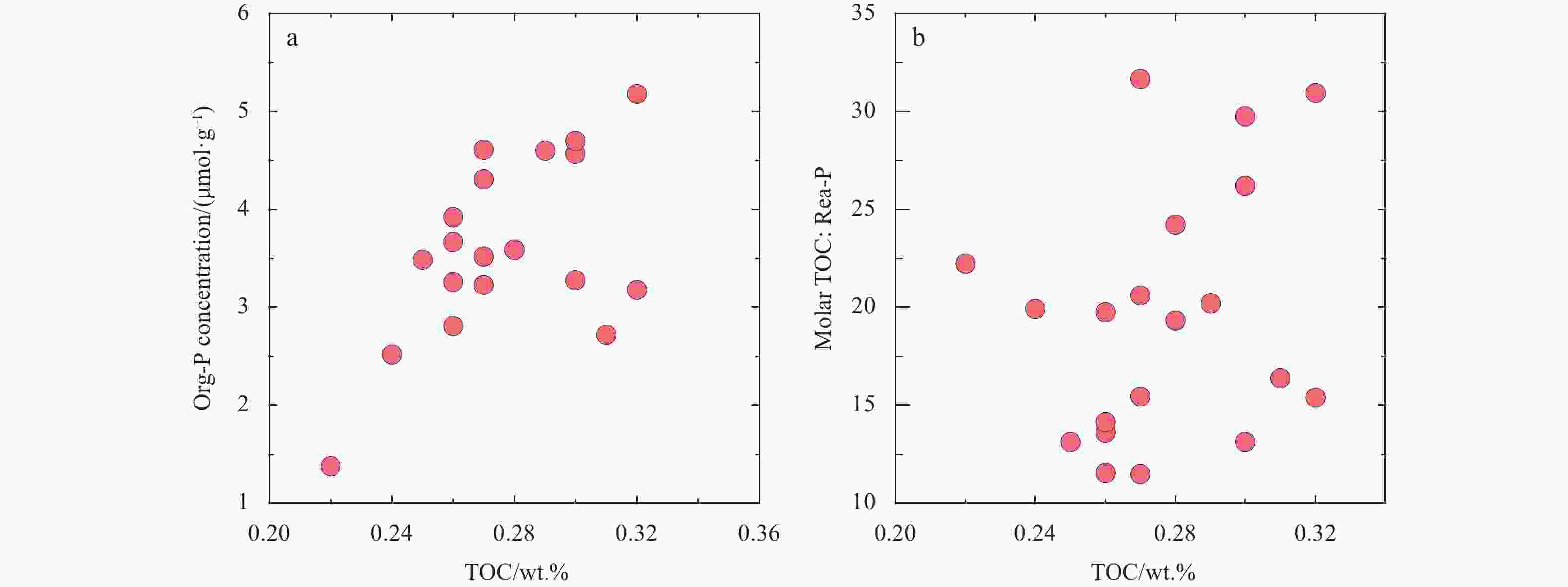
Abstract: The concentrations of five forms of phosphorus (P) including exchangeable or loosely adsorbed P (Ex-P), Fe-bound P (Fe-P), authigenic P (Auth-P), detrital P (Det-P), and organic P (Org-P) from the basin among the Marcus-Wake seamounts (19.4°–24°N, 156.5°–161.5°E) in the western Pacific Ocean were quantified using a sequential extraction method (SEDEX) to investigate the distribution and sources of different P species. Concentrations of total P (TP) varied from 14.0 μmol/g to 44.1 μmol/g, with an average of (32.4±7.7) μmol/g. Inorganic phosphorus, which was the major chemical form of sedimentary P, ranged from 12.6 μmol/g to 40.6 μmol/g, while the concentration of Org-P varied between 1.38 μmol/g and 5.18 μmol/g, accounting for 83.4%–93.4% and 6.6%–16.6% of the TP, respectively. The relative proportions of the five P species followed the order of Det-P>Auth-P>Org-P>Fe-P>Ex-P. On average, Det-P was the major P sink resulted from the atmospheric input and accounted for approximately 58.9%±12.4% of the TP. Auth-P and Org-P comprised 22.8%±11.4% and 11.5%±3.0% of the TP, respectively, while Fe-P accounted for 5.1%±2.6%. Lastly, Ex-P comprised 1.6%±0.3% of the TP. Org-P exhibited a negative correlation with Fe-P and Auth-P, while Fe-P showed a positive correlation with Auth-P. This indicated that the formation of Fe-P and Auth-P was at the expense of the regeneration or remineralization of Org-P during early diagenesis. High concentrations of Det-P and Auth-P as well as a low ratio of total organic C to reactive P (TOC/Rea-P) suggested that the aeolian input may play a significant role in sedimentary P budget in the study area. Additionally, well-oxygenated bottom water and low sedimentation rate could be responsible for the low TOC/Org-P ratio in the sediment.
-
Key words:
- phosphorus speciation /
- SEDEX /
- sediments /
- western Pacific Ocean
-
Figure 1. Potential atmospheric P sources and sampling locations in the western Pacific Ocean. The white arrows show the combined effects of Asian winter monsoon and westerly jet stream (modified based on Miyazaki et al. (2016)). The yellow lines highlight the spreading of the Antarctic Bottom Water (Emery, 2001; Kawabe and Fujio, 2010).
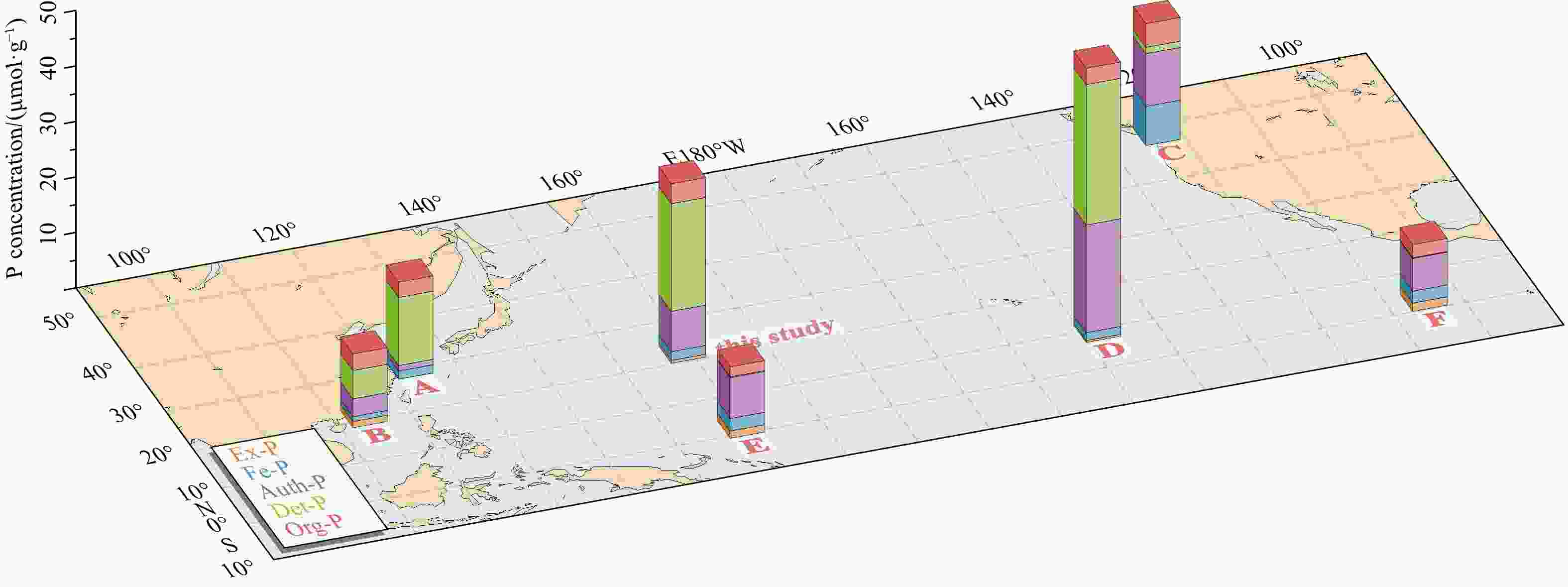
Figure 4. Variations in the concentration and speciation of five P phases in the Pacific Ocean based on our data and other studies (data from A. Fang et al. (2007); B. Yang et al. (2018); C. Filippelli (2001); D. Ni et al. (2015); E. ODP 130, and F. ODP 138 from Filippelli and Delaney (1996)).
Table 1. Sampling locations, water depths, and P concentrations (µmol/g) of total phosphorus (TP), total inorganic P (TIP), and total organic P (TOP) in dried sediment
Station Latitude Longitude Depth/m TP/(μmol·g−1) TIP/(μmol·g−1) TOP/(μmol·g−1) BC1601 19.80°N 158.54°E 5 648 43.3 40.1 3.18 BC1602 19.94°N 159.46°E 5 502 42.8 40.0 2.81 BC1603a 20.43°N 156.97°E 5 094 34.2 31.5 2.72 BC1604 20.43°N 157.20°E 5 467 14.0 12.6 1.38 BC1605 20.83°N 158.94°E 5 574 19.9 17.4 2.52 BC1606 20.39°N 158.47°E 5 574 37.5 34.2 3.23 BC1607 20.46°N 160.40°E 5 123 37.9 34.6 3.26 BC1608 20.24°N 160.34°E 4 934 44.1 40.6 3.52 BC1609 20.71°N 160.08°E 5 645 33.8 30.3 3.49 BC1610 20.93°N 160.85°E 4 993 37.3 32.7 4.61 BC1611 21.12°N 159.18°E 5 640 29.7 26.1 3.59 BC1612a 21.69°N 158.30°E 5 149 37.1 33.1 3.92 BC1613 22.15°N 158.98°E 5 293 31.6 27.1 4.57 BC1614b 21.99°N 159.75°E 5 321 24.1 20.8 3.28 BC1615 22.88°N 160.55°E 5 564 25.3 21.7 3.59 BC1616 22.52°N 161.13°E 5 052 29.2 24.9 4.31 BC1617 23.18°N 161.09°E 5 371 29.8 26.1 3.67 BC1618 23.88°N 160.13°E 5 528 30.3 25.6 4.70 BC1620 22.58°N 158.34°E 5 443 34.1 29.5 4.60 BC1621a 22.22°N 157.03°E 5 310 31.3 26.1 5.18 Table 2. Details of the sequential extraction procedure
Step Extraction Target 1 10 mL of 1 mol/L MgCl2 (pH 8), shaking for 4 h exchangeable or loosely adsorbed P (Ex-P) 2 10 mL of BD (0.11 mol/L NaHCO3+0.11 mol/L Na2S2O4, pH 7), shaking for 6 h reactive Fe-bound P (Fe-P) 3 10 mL of HAc/NaAc (pH 4), shaking for 6 h authigenic P (Auth-P) 4 10 mL of 1 mol/L HCl, shaking for 16 h detrital P (Det-P) 5 ashing at 550°C for 2 h, 10 mL of 1 mol/L HCl, shaking for 24 h organic P (Org-P) Table 3. Concentrations of different sedimentary P phases including exchangeable or loosely adsorbed P (Ex-P), Fe-bound P (Fe-P), authigenic P (Auth-P), detrital P (Det-P), and organic P (Org-P) (all in μmol/g) and total organic carbon (TOC) (in mass percent, wt.%), as well as molar ratio of TOC : Org-P and TOC : Rea-P in the basin of the Marcus-Wack seamounts
Station Ex-P Fe-P Auth-P Det-P Org-P TOC/% TOC : Org-P TOC : Rea-P BC1601 0.59 2.28 11.26 26.00 3.18 0.32 83.9 15.4 BC1602 0.76 2.26 12.90 24.11 2.81 0.26 77.1 11.6 BC1603a 0.47 2.11 10.45 18.44 2.72 0.31 95.0 16.4 BC1604 0.19 1.31 5.36 5.73 1.38 0.22 132.8 22.2 BC1605 0.50 1.86 5.16 9.87 2.52 0.24 79.4 19.9 BC1606 0.57 2.23 8.53 22.92 3.23 0.27 69.7 15.4 BC1607 0.54 1.94 10.18 21.96 3.26 0.26 66.5 13.6 BC1608 0.69 2.41 12.94 24.54 3.52 0.27 63.9 11.5 BC1609 0.56 2.50 9.31 17.89 3.49 0.25 59.7 13.1 BC1610 0.65 0.74 4.91 26.43 4.61 0.27 48.8 20.6 BC1611 0.51 1.94 6.03 17.66 3.59 0.28 65.0 19.3 BC1612a 0.49 2.41 8.50 21.74 3.92 0.26 55.3 14.1 BC1613 0.66 0.92 3.38 22.10 4.57 0.30 54.7 26.2 BC1614b 0.47 2.13 13.13 5.06 3.28 0.30 76.2 13.2 BC1615 0.47 1.72 3.85 15.67 3.59 0.28 65.0 24.2 BC1616 0.47 0.58 1.74 22.07 4.31 0.27 52.2 31.7 BC1617 0.47 0.97 5.86 18.83 3.67 0.26 59.0 19.7 BC1618 0.47 0.38 2.85 21.93 4.70 0.30 53.2 29.8 BC1620 0.47 0.53 6.36 22.14 4.60 0.29 52.5 20.2 BC1621a 0.47 0.47 2.49 22.68 5.18 0.32 51.5 31.0 Average 0.52 1.58 7.26 19.39 3.61 0.28 68.1 19.5 SD 0.12 0.76 3.68 6.11 0.90 0.03 19.7 6.4 -
[1] Acharya S S, Panigrahi M K, Kurian J, et al. 2016. Speciation of phosphorus in the continental shelf sediments in the Eastern Arabian Sea. Continental Shelf Research, 115: 65–75. doi: 10.1016/j.csr.2016.01.005 [2] Algeo T J, Ingall E. 2007. Sedimentary Corg: P ratios, paleocean ventilation, and Phanerozoic atmospheric pO2. Palaeogeography, Palaeoclimatology, Palaeoecology, 256(3–4): 130–155, [3] Anderson L D, Delaney M L, Faul K L. 2001. Carbon to phosphorus ratios in sediments: Implications for nutrient cycling. Global Biogeochemical Cycles, 15(1): 65–79. doi: 10.1029/2000gb001270 [4] Anderson L D, Faul K L, Paytan A. 2010. Phosphorus associations in aerosols: What can they tell us about P bioavailability?. Marine Chemistry, 120(1–4): 44–56. doi: 10.1016/j.marchem.2009.04.008 [5] Aydin I, Aydin F, Saydut A, et al. 2009. A sequential extraction to determine the distribution of phosphorus in the seawater and marine surface sediment. Journal of Hazardous Materials, 168(2–3): 664–669. doi: 10.1016/j.jhazmat.2009.02.095 [6] Babu C P, Nath B N. 2005. Processes controlling forms of phosphorus in surficial sediments from the eastern Arabian Sea impinged by varying bottom water oxygenation conditions. Deep-Sea Research Part II: Topical Studies in Oceanography, 52(14–15): 1965–1980. doi: 10.1016/j.dsr2.2005.06.004 [7] Baturin G N. 1988. Disseminated phosphorus in oceanic sediments — A review. Marine Geology, 84(1–2): 95–104. doi: 10.1016/0025-3227(88)90127-2 [8] Benitez-Nelson C R. 2000. The biogeochemical cycling of phosphorus in marine systems. Earth-Science Reviews, 51(1–4): 109–135. doi: 10.1016/s0012-8252(00)00018-0 [9] Berner R A. 1973. Phosphate removal from sea water by adsorption on volcanogenic ferric oxides. Earth and Planetary Science Letters, 18(1): 77–86. doi: 10.1016/0012-821X(73)90037-X [10] Berner R A, Rao J L. 1994. Phosphorus in sediments of the Amazon River and estuary: Implications for the global flux of phosphorus to the sea. Geochimica et Cosmochimica Acta, 58(10): 2333–2339. doi: 10.1016/0016-7037(94)90014-0 [11] Broecker W S. 1982. Glacial to interglacial changes in ocean chemistry. Progress in Oceanography, 11(2): 151–197. doi: 10.1016/0079-6611(82)90007-6 [12] Chen Hungyu, Fang T H, Preston M R, et al. 2006. Characterization of phosphorus in the aerosol of a coastal atmosphere: Using a sequential extraction method. Atmospheric Environment, 40(2): 279–289. doi: 10.1016/j.atmosenv.2005.09.051 [13] Delaney M L. 1998. Phosphorus accumulation in marine sediments and the oceanic phosphorus cycle. Global Biogeochemical Cycles, 12(4): 563–572. doi: 10.1029/98GB02263 [14] de Lange G J. 1992. Distribution of various extracted phosphorus compounds in the interbedded turbiditic/pelagic sediments of the Madeira Abyssal Plain, eastern North Atlantic. Marine Geology, 109(1–2): 115–139. doi: 10.1016/0025-3227(92)90224-6 [15] Eijsink L M, Krom M D, Herut B. 2000. Speciation and burial flux of phosphorus in the surface sediments of the Eastern Mediterranean. American Journal of Science, 300(6): 483–503. doi: 10.2475/ajs.300.6.483 [16] Emery W J. 2001. Water types and water masses. In: Steele J H, Thorpe S A, Turekian K K, eds. Encyclopedia of Ocean Sciences. San Diego, CA: Academic Press, 3179–3187 [17] Fang T H, Chen J L, Huh C A. 2007. Sedimentary phosphorus species and sedimentation flux in the East China Sea. Continental Shelf Research, 27(10–11): 1465–1476. doi: 10.1016/j.csr.2007.01.011 [18] Faul K L, Paytan A, Delaney M L. 2005. Phosphorus distribution in sinking oceanic particulate matter. Marine Chemistry, 97(3–4): 307–333. doi: 10.1016/j.marchem.2005.04.002 [19] Filippelli G M. 2001. Carbon and phosphorus cycling in anoxic sediments of the Saanich Inlet, British Columbia. Marine Geology, 174(1–4): 307–321. doi: 10.1016/s0025-3227(00)00157-2 [20] Filippelli G M, Delaney M L. 1994. The oceanic phosphorus cycle and continental weathering during the Neogene. Paleoceanography, 9(5): 643–652. doi: 10.1029/94pa01453 [21] Filippelli G M, Delaney M L. 1996. Phosphorus geochemistry of equatorial Pacific sediments. Geochimica et Cosmochimica Acta, 60(9): 1479–1495. doi: 10.1016/0016-7037(96)00042-7 [22] Flaum J A. 2008. Investigation of phosphorus cycle dynamics associated with organic carbon burial in modern (North Pacific) and ancient (Devonian and Cretaceous) marine systems; strengths and limitations of sequentially extracted (SEDEX) phosphorus data [dissertation]. Evanston: Northwestern University [23] Föllmi K B. 1996. The phosphorus cycle, phosphogenesis and marine phosphate-rich deposits. Earth-Science Reviews, 40(1–2): 55–124. doi: 10.1016/0012-8252(95)00049-6 [24] Froelich P N. 1988. Kinetic control of dissolved phosphate in natural rivers and estuaries: A primer on the phosphate buffer mechanism. Limnology and Oceanography, 33(4part2): 649–668. doi: 10.4319/lo.1988.33.4part2.0649 [25] Froelich P N, Arthur M A, Burnett W C, et al. 1988. Early diagenesis of organic matter in Peru continental margin sediments: Phosphorite precipitation. Marine Geology, 80(3–4): 309–343. doi: 10.1016/0025-3227(88)90095-3 [26] Gächter R, Meyer J S. 1993. The role of microorganisms in mobilization and fixation of phosphorus in sediments. Hydrobiologia, 253(1–3): 103–121. doi: 10.1007/bf00050731 [27] Garrison T, Ellis R. 2015. Oceanography: An Invitation to Marine Science. 9th ed. Boston, MA: Cengage Learning, 139–165, 255 [28] Guo Boshu, Yang Hongwei, Li Yan. 2011. The speciation of phosphorus in the sand particles in Western Inner Mongolia. In: Proceedings of the Second International Conference on Mechanic Automation and Control Engineering. Inner Mongolia: IEEE, 2755–2757 [29] Hansen H P, Koroleff F. 1999. Determination of nutrients. In: Grasshoff K, Kremling K, Ehrhardt M, eds. Methods of Seawater Analysis. Weinheim: Wiley, 159–228 [30] Hashihama F, Furuya K, Kitajima S, et al. 2009. Macro-scale exhaustion of surface phosphate by dinitrogen fixation in the western North Pacific. Geophysical Research Letters, 36(3): L03610. doi: 10.1029/2008GL036866 [31] Herbert R J, Krom M D, Carslaw K S, et al. 2018. The effect of atmospheric acid processing on the global deposition of bioavailable phosphorus from dust. Global Biogeochemical Cycles, 32(9): 1367–1385. doi: 10.1029/2018GB005880 [32] Herut B, Collier R, Krom M D. 2002. The role of dust in supplying nitrogen and phosphorus to the southeast Mediterranean. Limnology and Oceanography, 47(3): 870–878. doi: 10.4319/lo.2002.47.3.0870 [33] Iijima K, Yasukawa K, Fujinaga K, et al. 2016. Discovery of extremely REY-rich mud in the western North Pacific Ocean. Geochemical Journal, 50(6): 557–573. doi: 10.2343/geochemj.2.0431 [34] Ingall D, Bustin R M, Van Cappellen P. 1993. Influence of water column anoxia on the burial and preservation of carbon and phosphorus in marine shales. Geochimica et Cosmochimica Acta, 57(2): 303–316. doi: 10.1016/0016-7037(93)90433-W [35] Ingall E, Jahnke R. 1994. Evidence for enhanced phosphorus regeneration from marine sediments overlain by oxygen depleted waters. Geochimica et Cosmochimica Acta, 58(11): 2571–2575. doi: 10.1016/0016-7037(94)90033-7 [36] Ingall E D, Van Cappellen P. 1990. Relation between sedimentation rate and burial of organic phosphorus and organic carbon in marine sediments. Geochimica et Cosmochimica Acta, 54(2): 373–386. doi: 10.1016/0016-7037(90)90326-g [37] Jiang Cuihong, Hu Jiwei, Huang Xianfei, et al. 2011. Phosphorus speciation in sediments of Lake Hongfeng, China. Chinese Journal of Oceanology and Limnology, 29(1): 53–62. doi: 10.1007/s00343-011-9047-4 [38] Jickells T D, An Z S, Andersen K K, et al. 2005. Global iron connections between desert dust, ocean biogeochemistry, and climate. Science, 308(5718): 67–71. doi: 10.1126/science.1105959 [39] Jones C E, Halliday A N, Rea D K, et al. 2000. Eolian inputs of lead to the North Pacific. Geochimica et Cosmochimica Acta, 64(8): 1405–1416. doi: 10.1016/s0016-7037(99)00439-1 [40] Kang Xuming, Song Jinming, Yuan Huamao, et al. 2017. Phosphorus speciation and its bioavailability in sediments of the Jiaozhou Bay. Estuarine, Coastal and Shelf Science, 188: 127–136, [41] Kawabe M, Fujio S. 2010. Pacific Ocean circulation based on observation. Journal of Oceanography, 66(3): 389–403. doi: 10.1007/s10872-010-0034-8 [42] Kong Fanping, Dong Qing, Xiang Kunsheng, et al. 2019. Spatiotemporal variability of remote sensing ocean net primary production and major forcing factors in the tropical eastern Indian and western Pacific Ocean. Remote Sensing, 11(4): 391. doi: 10.3390/rs11040391 [43] Kraal P, Slomp C P, Reed D C, et al. 2012. Sedimentary phosphorus and iron cycling in and below the oxygen minimum zone of the northern Arabian Sea. Biogeosciences, 9(7): 2603–2624. doi: 10.5194/bg-9-2603-2012 [44] Kujawinski E B. 2011. The impact of microbial metabolism on marine dissolved organic matter. Annual Review of Marine Science, 3(1): 567–599. doi: 10.1146/annurev-marine-120308-081003 [45] Kyte F T, Leinen M, Heath G R, et al. 1993. Cenozoic sedimentation history of the central North Pacific: Inferences from the elemental geochemistry of core LL44-GPC3. Geochimica et Cosmochimica Acta, 57(8): 1719–1740. doi: 10.1016/0016-7037(93)90109-a [46] Lin Peng, Klump J V, Guo Laodong. 2016. Dynamics of dissolved and particulate phosphorus influenced by seasonal hypoxia in Green Bay, Lake Michigan. Science of the Total Environment, 541: 1070–1082. doi: 10.1016/j.scitotenv.2015.09.118 [47] Linsy P, Nath B N, Mascarenhas-Pereira M B L, et al. 2018. Distribution and diagenesis of phosphorus in the deep-sea sediments of the Central Indian Basin. Journal of Geophysical Research: Oceans, 123(11): 7963–7982. doi: 10.1029/2018JC014386 [48] Łukawska-Matuszewska K, Bolałek J. 2008. Spatial distribution of phosphorus forms in sediments in the Gulf of Gdańsk (southern Baltic Sea). Continental Shelf Research, 28(7): 977–990. doi: 10.1016/j.csr.2008.01.009 [49] Łukawska-Matuszewska K, Burska D. 2011. Phosphate exchange across the sediment-water interface under oxic and hypoxic/anoxic conditions in the southern Baltic Sea. Oceanological and Hydrobiological Studies, 40(2): 57–71. doi: 10.2478/s13545-011-0017-4 [50] Maher B A, Prospero J M, Mackie D, et al. 2010. Global connections between aeolian dust, climate and ocean biogeochemistry at the present day and at the last glacial maximum. Earth-Science Reviews, 99(1–2): 61–97. doi: 10.1016/j.earscirev.2009.12.001 [51] Mahowald N, Jickells T D, Baker A R, et al. 2008. Global distribution of atmospheric phosphorus sources, concentrations and deposition rates, and anthropogenic impacts. Global Biogeochemical Cycles, 22(4): GB4026. doi: 10.1029/2008gb003240 [52] Martiny A C, Lomas M W, Fu Weiwei, et al. 2019. Biogeochemical controls of surface ocean phosphate. Science Advances, 5(8): eaax0341. doi: 10.1126/sciadv.aax0341 [53] März C, Poulton S W, Wagner T, et al. 2014. Phosphorus burial and diagenesis in the central Bering Sea (Bowers Ridge, IODP Site U1341): Perspectives on the marine P cycle. Chemical Geology, 363: 270–282. doi: 10.1016/j.chemgeo.2013.11.004 [54] Miyazaki T, Kimura J I, Katakuse M. 2016. Geochemical records from loess deposits in Japan over the last 210 kyr: Lithogenic source changes and paleoclimatic indications. Geochemistry, Geophysics, Geosystems, 17(7): 2745–2761, [55] Monbet P, Brunskill G J, Zagorskis I, et al. 2007. Phosphorus speciation in the sediment and mass balance for the central region of the Great Barrier Reef continental shelf (Australia). Geochimica et Cosmochimica Acta, 71(11): 2762–2779. doi: 10.1016/j.gca.2007.03.025 [56] Moody J B, Chaboudy L R, Worsley T R. 1988. Pacific pelagic phosphorus accumulation during the last 10 M.Y. Paleoceanography, 3(1): 113–136. doi: 10.1029/pa003i001p00113 [57] Murray R W, Leinen M. 1993. Chemical transport to the seafloor of the equatorial Pacific Ocean across a latitudinal transect at 135°W: Tracking sedimentary major, trace, and rare earth element fluxes at the Equator and the Intertropical Convergence Zone. Geochimica et Cosmochimica Acta, 57(17): 4141–4163. doi: 10.1016/0016-7037(93)90312-K [58] Nenes A, Krom M D, Mihalopoulos N, et al. 2011. Atmospheric acidification of mineral aerosols: a source of bioavailable phosphorus for the oceans. Atmospheric Chemistry and Physics, 11(13): 6265–6272. doi: 10.5194/acp-11-6265-2011 [59] Ni Jianyu, Lin Peng, Zhen Yang, et al. 2015. Distribution, source and chemical speciation of phosphorus in surface sediments of the central Pacific Ocean. Deep-Sea Research Part I: Oceanographic Research Papers, 105: 74–82. doi: 10.1016/j.dsr.2015.08.008 [60] Nishi K, Usui A, Nakasato Y, et al. 2017. Formation age of the dual structure and environmental change recorded in hydrogenetic ferromanganese crusts from Northwest and Central Pacific seamounts. Ore Geology Reviews, 87: 62–70. doi: 10.1016/j.oregeorev.2016.09.004 [61] Paytan A, Cade-Menun B J, McLaughlin K, et al. 2003. Selective phosphorus regeneration of sinking marine particles: evidence from 31P-NMR. Marine Chemistry, 82(1–2): 55–70. doi: 10.1016/s0304-4203(03)00052-5 [62] Paytan A, McLaughlin K. 2007. The oceanic phosphorus cycle. Chemical Reviews, 107(2): 563–576. doi: 10.1021/cr0503613 [63] Prospero J M, Ginoux P, Torres O, et al. 2002. Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Reviews of Geophysics, 40(1): 1002. doi: 10.1029/2000rg000095 [64] Reinhard C T, Planavsky N J, Gill B C, et al. 2017. Evolution of the global phosphorus cycle. Nature, 541(7637): 386–389. doi: 10.1038/nature20772 [65] Ruttenberg K C. 1992. Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnology and Oceanography, 37(7): 1460–1482. doi: 10.4319/lo.1992.37.7.1460 [66] Ruttenberg K C. 2014. The global phosphorus cycle. In: Turekian K K, ed. Treatise on Geochemistry. 2th ed. Oxford: Elsevier, 499–558 [67] Ruttenberg K C, Berner R A. 1993. Authigenic apatite formation and burial in sediments from non-upwelling, continental margin environments. Geochimica et Cosmochimica Acta, 57(5): 991–1007. doi: 10.1016/0016-7037(93)90035-u [68] Seibold E, Berger W. 2017. The Sea Floor: An Introduction to Marine Geology. 4th ed. Berlin: Springer [69] Shi Jinhui, Wang Nan, Gao Huiwang, et al. 2019. Phosphorus solubility in aerosol particles related to particle sources and atmospheric acidification in Asian continental outflow. Atmospheric Chemistry and Physics, 19(2): 847–860. doi: 10.5194/acp-19-847-2019 [70] Slomp C P. 2011. Phosphorus cycling in the estuarine and coastal zones: Sources, sinks, and transformations. In: Wolanski E, McLusky D S, eds. Treatise on Estuarine and Coastal Science. London: Academic Press, 201–229 [71] Tanaka E, Nakamura K, Yasukawa K, et al. 2020. Chemostratigraphy of deep-sea sediments in the western North Pacific Ocean: Implications for genesis of mud highly enriched in rare-earth elements and yttrium. Ore Geology Reviews, 119: 103392. doi: 10.1016/j.oregeorev.2020.103392 [72] Tsandev I, Reed D C, Slomp C P. 2012. Phosphorus diagenesis in deep-sea sediments: Sensitivity to water column conditions and global scale implications. Chemical Geology, 330–331: 127–139, [73] Tyrrell T. 1999. The relative influences of nitrogen and phosphorus on oceanic primary production. Nature, 400(6744): 525–531. doi: 10.1038/22941 [74] Vink S, Chambers R M, Smith S V. 1997. Distribution of phosphorus in sediments from Tomales Bay, California. Marine Geology, 139(1–4): 157–179. doi: 10.1016/s0025-3227(96)00109-0 [75] Wessel P, Smith W H F, Scharroo R, et al. 2013. Generic mapping tools: Improved version released. Eos, Transactions American Geophysical Union, 94(45): 409–410, [76] Yang Bin, Liu Sumei, Zhang Guoling. 2018. Geochemical characteristics of phosphorus in surface sediments from the continental shelf region of the northern South China Sea. Marine Chemistry, 198: 44–55. doi: 10.1016/j.marchem.2017.11.001 [77] Yang Zifei, Qian Qiankun, Chen Min, et al. 2020. Enhanced but highly variable bioturbation around seamounts in the northwest Pacific. Deep-Sea Research Part I: Oceanographic Research Papers, 156: 103190. doi: 10.1016/j.dsr.2019.103190 [78] Yasukawa K, Ohta J, Miyazaki T, et al. 2019. Statistic and isotopic characterization of deep-sea sediments in the western North Pacific Ocean: Implications for genesis of the sediment extremely enriched in rare earth elements. Geochemistry, Geophysics, Geosystems, 20(7): 3402–3430, [79] Zhang Jiazhong, Guo Laodong, Fischer C J. 2010. Abundance and chemical speciation of phosphorus in sediments of the Mackenzie River Delta, the Chukchi Sea and the Bering Sea: Importance of detrital apatite. Aquatic Geochemistry, 16(3): 353–371. doi: 10.1007/s10498-009-9081-4 [80] Zhang Jiazhong, Huang Xiaolan. 2007. Relative importance of solid-phase phosphorus and iron on the sorption behavior of sediments. Environment Science & Technology, 41(8): 2789–2795. doi: 10.1021/es061836q [81] Zhao Wancang, Sun Youbin, Balsam W, et al. 2015. Clay-sized Hf-Nd-Sr isotopic composition of Mongolian dust as a fingerprint for regional to hemispherical transport. Geophysical Research Letters, 42(13): 5661–5669. doi: 10.1002/2015GL064357 [82] Zhou Lei, Kyte F T. 1992. Sedimentation history of the South Pacific pelagic clay province over the last 85 million years inferred from the geochemistry of Deep Sea Drilling Project Hole 596. Paleoceanography, 7(4): 441–465. doi: 10.1029/92pa01063 -





 下载:
下载: