Benthic microbial biogeography along the continental shelf shaped by substrates from the Changjiang River plume
-
Abstract: Coastal zones are active reactors of continental material including that transported by rivers via a series of microbiota-mediated reactions. Nevertheless, current knowledge of the ecology and functioning of the microbiota in coastal areas affected by large riverine inputs remains insufficient on a global scale. Here, an investigation on sediment microbial composition, including taxonomy and metabolic network, as well as their relationship with major benthic reaction substrates, namely carbon, nitrogen, sulphur and phosphorus, was conducted in the continental shelf affected by the spread of the Changjiang River plume. Surface sediment samples (48 samples) were collected during March 2018, obtaining a mean Operational Taxonomic Units (OTUs) number of 3 341. Proteobacteria, Acidobacteria and Actinobacteria were abundant phyla in the studied sediments. Bray-Curtis distance analysis classified the 48 samples into 4 clusters (MG1 to MG4) at the phylum-level. MG1 and MG2 are found near the river mouth, receiving substantial land-derived particles from the Changjiang River runoff. Particle-attached microbes may be settled in these regions and influenced the observed sediment microbial diversity and biomass, e.g., increased Crenarchaeota relative abundance. The relative enrichment of these two groups in heterotrophic microbes further suggests a reliance of benthic microbiota on substrates with terrestrial origin, particularly specialized on processing sulphur-rich substrates. Regions MG3 and MG4 are located in the outer margin of the area affected by the Changjiang River plume, mainly fed by settling pelagic particles from phytoplankton. Compared to MG1 and MG2, a significant increase in the abundance of Thaumarcheota (phylum-level) and Nitrosopumilus (genus-level) was found in MG3, suggesting nitrogen-related transformations as the key reactions to sustain microbial metabolism in this region. Coupled with the identified variations in the taxonomic composition, significant differences in the keystone taxa between MG1/MG2 and MG3/MG4 were identified via OTU co-occurrence analyses. A higher abundance of Actinobacteria, Thaumarchaeota and Acidobacteria in MG3 and MG4 reinforced the identified spatial variability in benthic metabolism and highlighted the significance of substrate inputs on the sediment microbial structure and biogeography.
-
Key words:
- benthic microbiota /
- biogeography /
- benthic substrate /
- Changjiang River plume /
- East China Sea /
- Yellow Sea
-
Figure 1. Spatial distributions of temperature (a) and salinity (b) in surface waters (1 m below sea surface) in the continental shelf affected by the Changjiang River plume during March 2018 as measured with CTD vertical profiles. Dots represent CTD measurement sites. Spatial distribution of the 48 sediment samples collected in the continental shelf affected by the Changjiang River plume in March 2018 (c). The Changjiang River outflow (CJ), Taiwan Warm Current (TWC) and the Yellow Sea Coastal Current (YSCC) are schematically represented based on Kim et al. (2018).
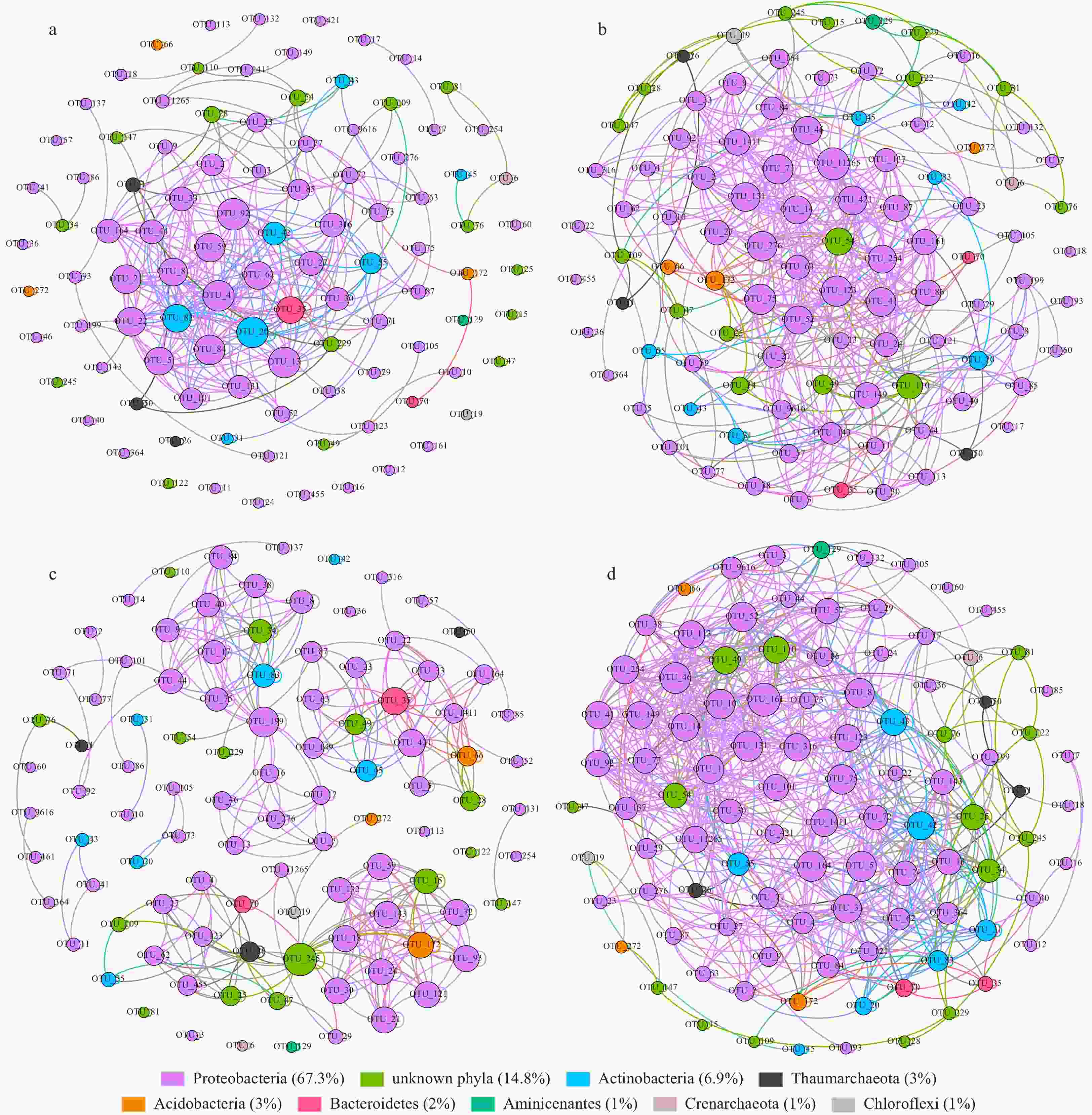
Figure 3. Network of co-occurring OTUs in MG1 (a), MG2 (b), MG3 (c) and MG4 (d), based on correlation analysis. The selection standards for strong and significant correlation are Spearman’s ρ>0.6 and p<0.01, respectively. The size of each node is proportional to the relative abundance of each OTU in the network; the color and thickness of each connection between two nodes (edge) are proportional to the value of Spearman’s correlation coefficients. The OTUs assigned to the same phylum were marked with the same color.
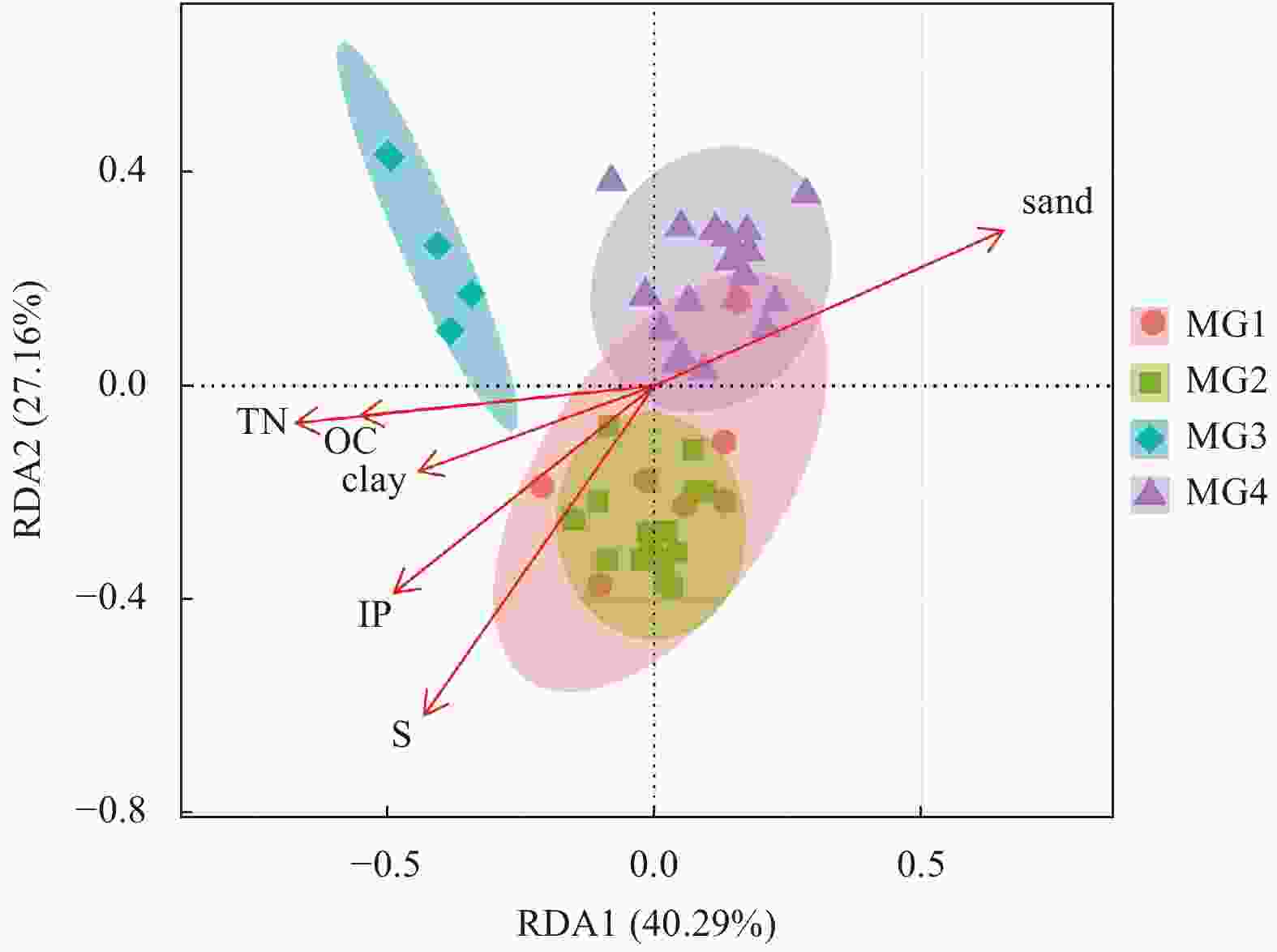
Figure 5. Relationships of overall community structure and environmental factors obtained through RDA analysis, including contents (according to mass fraction) of organic carbon (OC), total nitrogen (TN), sulfur (S), inorganic phosphorus (IP), and proportions of clay and sand in the continental shelf samples.
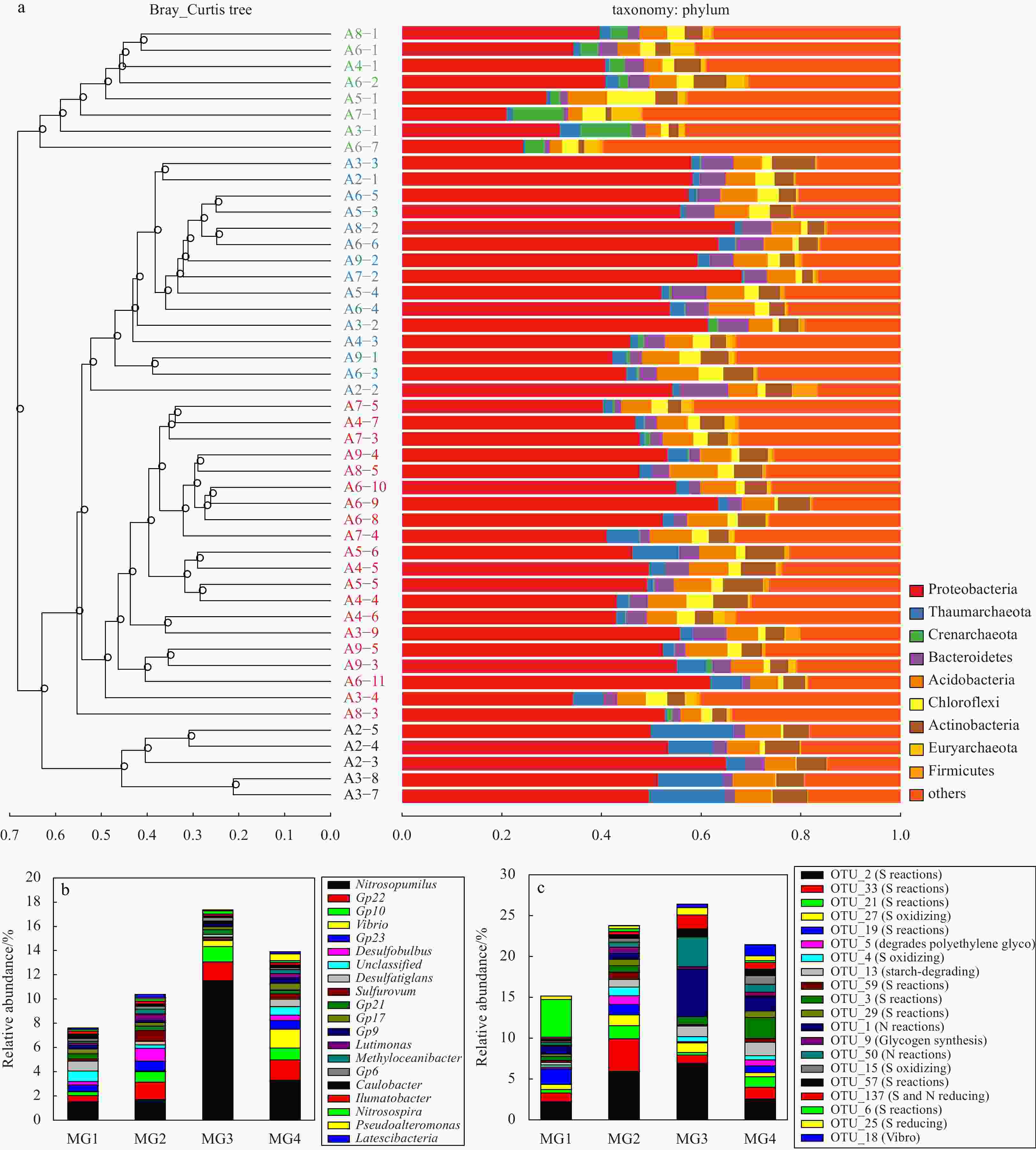
Table 1. Distribution of the top 20 most dominant OTUs, the closest uncultured bacteria 16S rRNA gene fragment and the closest cultured bacteria to these dominant OTUs
MG1 MG2 MG3 MG4 Closest uncultured bacteria
(accession numbers)Identity Closest cultured bacteria
(accession numbers)Identity OTU_2 2.21%±0.99% 5.92%±1.64% 6.92%±1.38% 2.56%±0.66% uncultured gamma proteobacterium (KU173695.1) 100% Woeseia oceani strain XK5
(NR_147719.1)96% OTU_33 1.06%±0.51% 4.00%±1.78% 1.00%±0.77% 1.43%±0.48% uncultured bacterium clone (KM356694.1) 100% Thioprofundum hispidum strain gps61 (NR_112620.1) 96% OTU_1 0.91%±0.90% 0.74%±0.36% 5.69%±2.51% 1.81%±1.33% uncultured marine archaeon (KJ504342.1) 100% Nitrosopumilus cobalaminigenes strain HCA1 (NR_159206.1) 99% OTU_3 0.52%±0.34% 0.79%±0.41% 0.95%±0.05% 2.55%±0.92% uncultured bacterium (MG949250.1) 100% Desulfatiglans parachlorophenolica strain DS (NR_126176.1) 90% OTU_13 0.30%±0.20% 0.93%±0.35% 1.28%±0.67% 1.64%±0.60% uncultured bacterium (MG002285.1) 100% Sandaracinus amylolyticus strain NOSO 4 (NR_118001.1) 89% OTU_21 0.43%±0.29% 1.60%±0.47% 0.32%±0.27% 1.29%±0.43% uncultured bacterium (KX097260.1) 100% Desulfobulbus propionicus strain DSM 2032 (NR_074930.1) 93% OTU_19 1.93%±1.20% 1.31%±0.73% 0.05%±0.04% 0.83%±0.41% uncultured Chloroflexi bacterium (GU061267.1) 100% Thermomarinilinea lacunifontana strain SW7 (NR_132293.1) 90% OTU_50 0.30%±0.22% 0.64%±0.40% 3.63%±1.50% 0.96%±0.70% uncultured marine group I crenarchaeote (JN590160.1) 100% Nitrosopumilus ureiphilus strain PS0 (NR_159208.1) 97% OTU_6 4.59%±3.39% 0.36%±0.47% 0.01%±0.01% 0.22%±0.30% uncultured archaeon (KX952634.1) 100% Thermogladius calderae strain 1633 (NR_148751.1) 85% OTU_27 0.61%±0.42% 1.32%±0.24% 1.18%±0.96% 0.50%±0.32% uncultured bacterium (JN621386.1) 99% Thiohalomonas nitratireducens strain HRHd 3sp (NR_043974.1) 93% OTU_5 0.21%±0.19% 1.06%±0.72% 0.08%±0.08% 0.72%±0.29% uncultured bacterium (KM203552.1) 100% Pelobacter venetianus strain Gra PEG 1 (NR_044779.1) 93% OTU_137 0.19%±0.1% 0.40%±0.20% 1.68%±0.73% 0.82%±0.46% uncultured bacterium (EU491352.1) 99% Thioprofundum lithotrophicum strain 106 (NR_112829.1) 94% OTU_4 0.28%±0.19% 1.04%±0.42% 0.66%±0.50% 0.53%±0.41% uncultured gamma proteobacterium (KR086607.1) 100% Thiohalobacter thiocyanaticus strain HRh1 (NR_116699.1) 93% OTU_29 0.29%±0.09% 0.77%±0.37% 0.15%±0.06% 0.82%±0.30% uncultured bacterium (KP292396.1) 100% Desulfonema magnum str. Montpellier strain 4be13(NR_025990.1) 93% OTU_15 0.21%±0.20% 0.48%±0.28% 0.10%±0.17% 1.05%±0.70% uncultured bacterium (HM598581.1) 100% Thermomarinilinea lacunifontana strain SW7 (NR_132293.1) 89% OTU_18 0.01%±0.01% 0.00%±0.00% 0.43%±0.40% 1.38%±0.47% no data ND Vibrio splendidus strain CH-105 (MH713000.1) 100% OTU_57 0.27%±0.12% 0.42%±0.12% 0.89%±0.22% 0.80%±0.33% uncultured bacterium (KX957641.1) 100% Desulfosarcina alkanivorans strain PL12 (NR_157797.1) 93% OTU_59 0.22%±0.13% 0.90%±0.29% 0.16%±0.19% 0.47%±0.23% uncultured bacterium (KR825168.1) 100% Desulfobulbus propionicus strain DSM 2032 (NR_074930.1) 91% OTU_25 0.44%±0.65% 0.35%±0.08% 0.92%±0.53% 0.58%±0.19% uncultured bacterium (EU734975.1) 100% Thermodesulfovibrio aggregans strain TGE-P1 (NR_040795.1) 85% OTU_9 0.18%±0.10% 0.74%±0.25% 0.33%±0.25% 0.49%±0.14% uncultured bacterium (KC631592.1) 100% Pseudohaliea rubra strain CM41_15a (NR_044426.1) 96% Table 2. The alpha diversity of the obtained four microbial groups in the sediments of the continental shelf affected by the Changjiang River plume
Richness Chao1 Shannon_2 Simpson Dominance Equitability MG1 3 337±386 3 338.49±386.00 9.38±0.50 0.007 7±0.005 0 0.990±0.005 0.80±0.04 MG2 3 463±495 3 464.57±495.00 9.20±0.40 0.009 1±0.003 0 0.990±0.003 0.78±0.03 MG3 3 391±516 3 392.54±516.00 9.38±0.30 0.006 8±0.005 0 0.990±0.005 0.80±0.03 MG4 2 789±397 2 791.24±397.00 8.55±0.30 0.014 0±0.004 0 0.980±0.004 0.75±0.02 Table 3. Average sediment organic carbon (OC), total nitrogen (TN), sulfur (S) and inorganic phosphorus (IP) contents together with sediment particle sizes (MPS) in the four microbial groups identified through Bray-Curtis distance
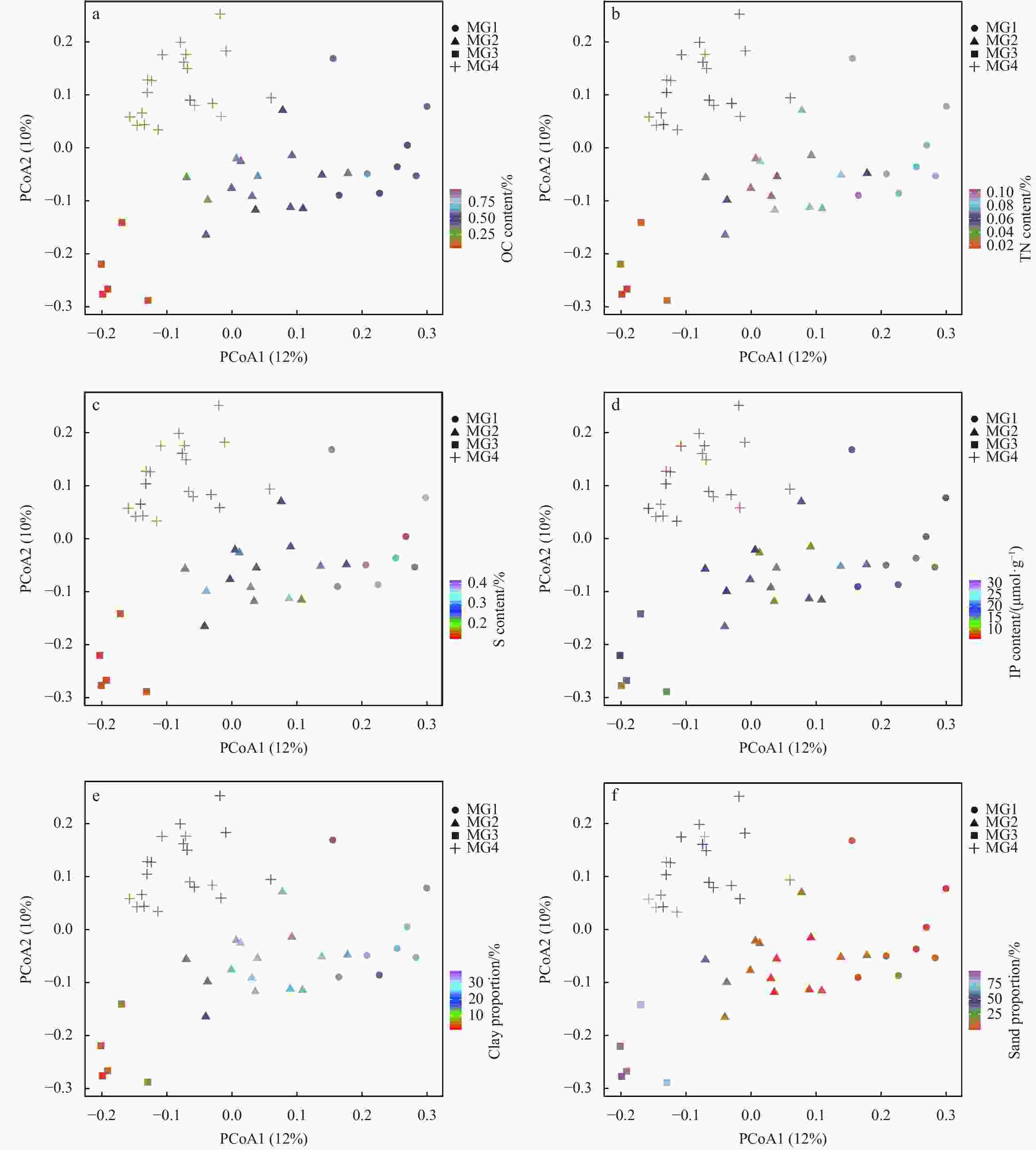
OC content/% TN content/% S content/% IP content/(μmol·g−1) MPS/µm Clay content/% Silt proportion/% Sand proportion/% MG1 0.5±0.1 0.1±0.0 0.38±0.02 17.8±3.1 9.1±2.7 30.4±4.5 64.0±3.6 5.6±4.3 MG2 0.5±0.2 0.1±0.0 0.28±0.04 18.5±4.4 12.8±8.4 27±5.9 63.3±6 9.7±10.6 MG3 0.1±0.0 0.0±0.0 0.14±0.00 14.9±3.3 239.0±43.4 4.8±2.3 9.1±4.9 86.1±7.1 MG4 0.3±0.1 0.1±0.0 0.22±0.04 16.4±6.0 76.8±54.4 18.7±4.0 30.0±8.4 51.3±11.7 -
[1] Aspila K I, Agemian H, Chau A S Y. 1976. A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst, 101(1200): 187–197. doi: 10.1039/AN9760100187 [2] Audry S, Blanc G, Schäfer J, et al. 2007. Effect of estuarine sediment resuspension on early diagenesis, sulfide oxidation and dissolved molybdenum and uranium distribution in the Gironde estuary, France. Chemical Geology, 238(3–4): 149–167. doi: 10.1016/j.chemgeo.2006.11.006 [3] Banerjee S, Schlaeppi K, van der Heijden M G A. 2018. Keystone taxa as drivers of microbiome structure and functioning. Nature Reviews Microbiology, 16(9): 567–576. doi: 10.1038/s41579-018-0024-1 [4] Bertics V J, Ziebis W. 2010. Bioturbation and the role of microniches for sulfate reduction in coastal marine sediments. Environmental Microbiology, 12(11): 3022–3034. doi: 10.1111/j.1462-2920.2010.02279.x [5] Bian Changwei, Jiang Wensheng, Quan Qi, et al. 2013. Distributions of suspended sediment concentration in the Yellow Sea and the East China Sea based on field surveys during the four seasons of 2011. Journal of Marine Systems, 121–122: 24–35. doi: 10.1016/j.jmarsys.2013.03.013 [6] Brandsma J, Martínez J M, Slagter H A, et al. 2013. Microbial biogeography of the North Sea during summer. Biogeochemistry, 113(1–3): 119–136 [7] Burchard H, Schuttelaars H M, Ralston D K. 2018. Sediment trapping in estuaries. Annual Review of Marine Science, 10(1): 371–395. doi: 10.1146/annurev-marine-010816-060535 [8] Caporaso J G, Bittinger K, Bushman F D, et al. 2010a. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics, 26(2): 266–267. doi: 10.1093/bioinformatics/btp636 [9] Caporaso J G, Kuczynski J, Stombaugh J, et al. 2010b. QIIME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5): 335–336. doi: 10.1038/nmeth.f.303 [10] Chang Yan, Müller M, Wu Ying, et al. 2020. Distribution and behaviour of dissolved selenium in tropical peatland-draining rivers and estuaries of Malaysia. Biogeosciences, 17(4): 1133–1145. doi: 10.5194/bg-17-1133-2020 [11] Chang Yan, Wu Ying, Zhang Jing, et al. 2021. Effects of algal blooms on selenium species dynamics: A case study in the Changjiang Estuary, China. Science of the Total Environment, 768: 144235. doi: 10.1016/j.scitotenv.2020.144235 [12] Chang Yan, Zhang Jing, Qu Jianguo, et al. 2016. The behavior of dissolved inorganic selenium in the Changjiang Estuary. Journal of Marine Systems, 154: 110–121. doi: 10.1016/j.jmarsys.2015.01.008 [13] Chen Wei, de Swart H E. 2018. Longitudinal variation in lateral trapping of fine sediment in tidal estuaries: observations and a 3D exploratory model. Ocean Dynamics, 68(3): 309–326. doi: 10.1007/s10236-018-1134-z [14] Chen Lianguo, Tsui M M P, Lam J C W, et al. 2019. Variation in microbial community structure in surface seawater from Pearl River Delta: Discerning the influencing factors. Science of the Total Environment, 660: 136–144. doi: 10.1016/j.scitotenv.2018.12.480 [15] Chen Ye, Li Siqi, Xu Xiaoqing, et al. 2021. Characterization of microbial communities in sediments of the South Yellow Sea. Journal of Oceanology and Limnology, 39(3): 846–864. doi: 10.1007/s00343-020-0106-6 [16] Chen Changsheng, Xue Pengfei, Ding Pingxing, et al. 2008. Physical mechanisms for the offshore detachment of the Changjiang Diluted Water in the East China Sea. Journal of Geophysical Research: Oceans, 113(C2): C02002. doi: 10.1029/2006JC003994 [17] Cleary D F R, Coelho F J R C, Oliveira V, et al. 2017. Sediment depth and habitat as predictors of the diversity and composition of sediment bacterial communities in an inter-tidal estuarine environment. Marine Ecology, 38(2): e12411. doi: 10.1111/maec.12411 [18] Cole J R, Wang Qiong, Fish J A, et al. 2014. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Research, 42(D1): D633–D642. doi: 10.1093/nar/gkt1244 [19] Dang Hongyue, Zhang Xiaoxia, Sun Jin, et al. 2008. Diversity and spatial distribution of sediment ammonia-oxidizing Crenarchaeota in response to estuarine and environmental gradients in the Changjiang Estuary and East China Sea. Microbiology, 154(7): 2084–2095. doi: 10.1099/mic.0.2007/013581-0 [20] Dong Yi, Zhao Yuan, Zhang Wenyan, et al. 2014. Bacterial diversity and community structure in the East China Sea by 454 sequencing of the 16S rRNA gene. Chinese Journal of Oceanology and Limnology, 32(3): 527–541. doi: 10.1007/s00343-014-3215-2 [21] Edgar R C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics, 26(19): 2460–2461. doi: 10.1093/bioinformatics/btq461 [22] Edgar R C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nature Methods, 10(10): 996–998. doi: 10.1038/NMETH.2604 [23] Edgar R C. 2016. SINTAX: a simple non-Bayesian taxonomy classifier for 16S and ITS sequences. bioRxiv. (2016-09-09)[2020-10-13]. http://europepmc.org/article/PPR/PPR33123. doi: 10.1101/074161 [24] Edgar R C, Flyvbjerg H. 2015. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics, 31(21): 3476–3482. doi: 10.1093/bioinformatics/btv401 [25] Feng Biwei, Li Xiaoran, Wang Jinhui, et al. 2009. Bacterial diversity of water and sediment in the Changjiang Estuary and coastal area of the East China Sea. FEMS Microbiology Ecology, 70(2): 236–248. doi: 10.1111/j.1574-6941.2009.00772.x [26] Fournier S, Lee T, Gierach M M. 2016. Seasonal and interannual variations of sea surface salinity associated with the Mississippi River plume observed by SMOS and Aquarius. Remote Sensing of Environment, 180: 431–439. doi: 10.1016/j.rse.2016.02.050 [27] Gao Jianhua, Wang Yaping, Pan Shaoming, et al. 2008. Spatial distributions of organic carbon and nitrogen and their isotopic compositions in sediments of the Changjiang Estuary and its adjacent sea area. Journal of Geographical Sciences, 18(1): 46–58. doi: 10.1007/s11442-008-0046-010.3321/j.issn:0375-5444.2007.09.009 [28] Gao Nan, Yang Guipeng, Zhang Honghai, et al. 2017. Temporal and spatial variations of three dimethylated sulfur compounds in the Changjiang Estuary and its adjacent area during summer and winter. Environmental Chemistry, 14(3): 160–177. doi: 10.1071/EN16158 [29] Gomez-Escribano J P, Alt S, Bibb M J. 2016. Next generation sequencing of actinobacteria for the discovery of novel natural products. Marine Drugs, 14(4): 78. doi: 10.3390/md14040078 [30] Gudasz C, Bastviken D, Steger K, et al. 2010. Temperature-controlled organic carbon mineralization in lake sediments. Nature, 466(7305): 478–481. doi: 10.1038/nature09186 [31] He Hui, Zhen Yu, Mi Tiezhu, et al. 2015. Community composition and distribution of sulfate- and sulfite-reducing prokaryotes in sediments from the Changjiang Estuary and adjacent East China Sea. Estuarine, 165: 75–85. doi: 10.1016/j.ecss.2015.09.005 [32] Hu Shaohua, Herner J D, Robertson W, et al. 2013. Emissions of polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs from heavy-duty diesel vehicles with DPF and SCR. Journal of the Air & Waste Management Association, 63(8): 984–996. doi: 10.1080/10962247.2013.795202 [33] Ibánhez J S P, Araujo M, Lefèvre N. 2016. The overlooked tropical oceanic CO2 sink. Geophysical Research Letters, 43(8): 3804–3812. doi: 10.1002/2016GL068020 [34] Inagaki F, Nunoura T, Nakagawa S, et al. 2006. Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin. Proceedings of the National Academy of Sciences of the United States of America, 103(8): 2815–2820. doi: 10.1073/pnas.0511033103 [35] Jiang Shan, Ibánhez J S P, Rocha C. 2018a. Influence of labile dissolved organic matter on nitrate reduction in a seepage face. Environmental Science and Pollution Research, 25(11): 10654–10667. doi: 10.1007/s11356-018-1302-1 [36] Jiang Shan, Jin Jie, Wu Ying, et al. 2021a. Response of nitrate processing to bio-labile dissolved organic matter supply under variable oxygen conditions in a sandy beach seepage face. Frontiers in Marine Science, 8:642143, doi: 10.3389/fmars.2021.642143 [37] Jiang Shan, Jin Jie, Zhang Guosen, et al. 2021b. Nitrate in the Changjiang diluted water: an isotopic evaluation on sources and reaction pathways. Journal of Oceanology and Limnology, 39(3): 830–845. doi: 10.1007/s00343-020-0149-8 [38] Jiang Shan, Kavanagh M, Ibánhez J S P, et al. 2021c. Denitrification-nitrification process in permeable coastal sediments: an investigation on the effect of salinity and nitrate availability using flow-through reactors. Acta Oceanologica Sinica, 40(10): 1–13. doi: 10.1007/s13131-021-1811-5 [39] Jiang Shan, Lu Haoliang, Liu Jingchun, et al. 2018b. Influence of seasonal variation and anthropogenic activity on phosphorus cycling and retention in mangrove sediments: A case study in China. Estuarine, 202: 134–144. doi: 10.1016/j.ecss.2017.12.011 [40] Jiang Shan, Müller M, Jin Jie, et al. 2019. Dissolved inorganic nitrogen in a tropical estuary in Malaysia: transport and transformation. Biogeosciences, 16(14): 2821–2836. doi: 10.5194/bg-16-2821-2019 [41] Jiang Shan, Zhang Yixue, Jin Jie, et al. 2020. Organic carbon in a seepage face of a subterranean estuary: Turnover and microbial interrelations. Science of the Total Environment, 725: 138220. doi: 10.1016/j.scitotenv.2020.138220 [42] Jiao Shuo, Liu Zhenshan, Lin Yanbing, et al. 2016. Bacterial communities in oil contaminated soils: Biogeography and co-occurrence patterns. Soil Biology and Biochemistry, 98: 64–73. doi: 10.1016/j.soilbio.2016.04.005 [43] Jiao Lijing, Wu Jianpeng, He Xiang, et al. 2018. Significant microbial nitrogen loss from denitrification and anammox in the land-sea interface of low permeable sediments. International Biodeterioration & Biodegradation, 135: 80–89. doi: 10.1016/j.ibiod.2018.10.002 [44] Jiao Nianzhi, Zhang Yao, Zeng Yonghui, et al. 2007. Ecological anomalies in the East China Sea: impacts of the Three Gorges Dam?. Water Research, 41(6): 1287–1293. doi: 10.1016/j.watres.2006.11.053 [45] Kim J, Cho H M, Kim G. 2018. Significant production of humic fluorescent dissolved organic matter in the continental shelf waters of the northwestern Pacific Ocean. Scientific Reports, 8(1): 4887. doi: 10.1038/s41598-018-23299-1 [46] Kochetkova T V, Kublanov I V, Toshchakov S V, et al. 2016. Thermogladius calderae gen. nov., sp. nov., an anaerobic, hyperthermophilic Crenarchaeote from a Kamchatka hot spring. International Journal of Systematic and Evolutionary Microbiology, 66(3): 1407–1412. doi: 10.1099/ijsem.0.000916 [47] Könneke M, Bernhard A E, de la Torre J R, et al. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature, 437(7058): 543–546. doi: 10.1038/nature03911 [48] Kuczynski J, Stombaugh J, Walters W A, et al. 2011. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Current Protocols in Bioinformatics, 36(1): 10.7.1–10.7.20. doi: 10.1002/0471250953.bi1007s36 [49] Kuypers M M M, Marchant H K, Kartal B. 2018. The microbial nitrogen-cycling network. Nature Reviews Microbiology, 16(5): 263–276. doi: 10.1038/nrmicro.2018.9 [50] Lefèvre N, Montes F M, Gaspar F L, et al. 2017. Net heterotrophy in the Amazon Continental Shelf changes rapidly to a sink of CO2 in the outer Amazon Plume. Frontiers in Marine Science, 4: 278. doi: 10.3389/fmars.2017.00278 [51] Leung M H Y, Chan K C K, Lee P K H. 2016. Skin fungal community and its correlation with bacterial community of urban Chinese individuals. Microbiome, 4(1): 46. doi: 10.1186/s40168-016-0192-z [52] Li Tongtong, Long Meng, Li Huan, et al. 2017. Multi-Omics analysis reveals a correlation between the host phylogeny, gut microbiota and metabolite profiles in cyprinid fishes. Frontiers in Microbiology, 8: 454. doi: 10.3389/fmicb.2017.00454 [53] Li Zhongqiao, Wu Ying, Yang Liyang, et al. 2020. Carbon isotopes and lignin phenols for tracing the floods during the past 70 years in the middle reach of the Changjiang River. Acta Oceanologica Sinica, 39(4): 33–41. doi: 10.1007/s13131-020-1543-y [54] Li Weijun, Zhou Shengzhen, Wang Xinfeng, et al. 2011. Integrated evaluation of aerosols from regional brown hazes over northern China in winter: Concentrations, sources, transformation, and mixing states. Journal of Geophysical Research: Atmospheres, 116(D9): D09301. doi: 10.1029/2010JD015099 [55] Liang Jiawei, Mai Wenning, Tang Jinfeng, et al. 2019. Highly effective treatment of petrochemical wastewater by a super-sized industrial scale plant with expanded granular sludge bed bioreactor and aerobic activated sludge. Chemical Engineering Journal, 360: 15–23. doi: 10.1016/j.cej.2018.11.167 [56] Liu Cheng, Hou Lijun, Liu Min, et al. 2019. Coupling of denitrification and anaerobic ammonium oxidation with nitrification in sediments of the Yangtze Estuary: Importance and controlling factors. Estuarine, 220: 64–72. doi: 10.1016/j.ecss.2019.02.043 [57] Liu Min, Xiao Tian, Wu Ying, et al. 2011. Temporal distribution of the archaeal community in the Changjiang Estuary hypoxia area and the adjacent East China Sea as determined by denaturing gradient gel electrophoresis and multivariate analysis. Canadian Journal of Microbiology, 57(6): 504–513. doi: 10.1139/w11-037 [58] Liu Tang, Zhang Anni, Wang Jiawen, et al. 2018. Integrated biogeography of planktonic and sedimentary bacterial communities in the Yangtze River. Microbiome, 6(1): 16. doi: 10.1186/s40168-017-0388-x [59] Løvdal T, Skjoldal E F, Heldal M, et al. 2008. Changes in morphology and elemental composition of Vibrio splendidus along a gradient from carbon-limited to phosphate-limited growth. Microbial Ecology, 55(1): 152–161. doi: 10.1007/s00248-007-9262-x [60] Mai Yongzhan, Lai Zini, Li Xinhui, et al. 2018. Structural and functional shifts of bacterioplanktonic communities associated with spatiotemporal gradients in river outlets of the subtropical Pearl River Estuary, South China. Marine Pollution Bulletin, 136: 309–321. doi: 10.1016/j.marpolbul.2018.09.013 [61] McCaig A E, Phillips C J, Stephen J R, et al. 1999. Nitrogen cycling and community structure of proteobacterial β-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Applied and Environmental Microbiology, 65(1): 213–220. doi: 10.1128/AEM.65.1.213-220.1999 [62] Navas-Molina J A, Peralta-Sánchez J M, González A, et al. 2013. Advancing our understanding of the human microbiome using QIIME. Methods in Enzymology, 531: 371–444. doi: 10.1016/B978-0-12-407863-5.00019-8 [63] Nold S C, Zhou Jizhong, Devol A H, et al. 2000. Pacific northwest marine sediments contain ammonia-oxidizing bacteria in the β subdivision of the Proteobacteria. Applied and Environmental Microbiology, 66(10): 4532–4535. doi: 10.1128/aem.66.10.4532-4535.2000 [64] Pallud C, van Cappellen P. 2006. Kinetics of microbial sulfate reduction in estuarine sediments. Geochimica et Cosmochimica Acta, 70(5): 1148–1162. doi: 10.1016/j.gca.2005.11.002 [65] Piza F F, Prado P I, Manfio G P. 2004. Investigation of bacterial diversity in Brazilian tropical estuarine sediments reveals high actinobacterial diversity. Antonie van Leeuwenhoek, 86(4): 317–328. doi: 10.1007/s10482-004-0162-5 [66] Qin Wei, Heal K R, Ramdasi R, et al. 2017. Nitrosopumilus maritimus gen. nov., sp. nov., Nitrosopumilus cobalaminigenes sp. nov., Nitrosopumilus oxyclinae sp. nov., and Nitrosopumilus ureiphilus sp. nov., four marine ammonia-oxidizing archaea of the phylum Thaumarchaeota. International Journal of Systematic and Evolutionary Microbiology, 67(12): 5067–5079. doi: 10.1099/ijsem.0.002416 [67] Schink B, Stieb M. 1983. Fermentative degradation of polyethylene glycol by a strictly anaerobic, gram-negative, nonsporeforming bacterium, Pelobacter venetianus sp. nov. Applied and Environmental Microbiology, 46(6): 1905–1913. doi: 10.1128/AEM.45.6.1905-1913.1983 [68] Sia E S A, Zhu Zhuoyi, Zhang Jing, et al. 2019. Biogeographical distribution of microbial communities along the Rajang River–South China Sea continuum. Biogeosciences, 16(21): 4243–4260. doi: 10.5194/bg-16-4243-2019 [69] Sintes E, Bergauer K, De Corte D, et al. 2013. Archaeal amoA gene diversity points to distinct biogeography of ammonia-oxidizing Crenarchaeota in the ocean. Environmental Microbiology, 15(5): 1647–1658. doi: 10.1111/j.1462-2920.2012.02801.x [70] Smith M W, Zeigler Allen L, Allen A E, et al. 2013. Contrasting genomic properties of free-living and particle-attached microbial assemblages within a coastal ecosystem. Frontiers in Microbiology, 4: 120. doi: 10.3389/fmicb.2013.00120 [71] Sunagawa S, Coelho L P, Chaffron S, et al. 2015. Structure and function of the global ocean microbiome. Science, 348(6237): 1261359. doi: 10.1126/science.1261359 [72] Wang Huan, Hu Zhangxi, Chai Zhaoyang, et al. 2020. Blooms of Prorocentrum donghaiense reduced the species diversity of dinoflagellate community. Acta Oceanologica Sinica, 39(4): 110–119. doi: 10.1007/s13131-020-1585-1 [73] Wang Wentao, Yu Zhiming, Wu Zaixing, et al. 2018. Rates of nitrification and nitrate assimilation in the Changjiang River Estuary and adjacent waters based on the nitrogen isotope dilution method. Continental Shelf Research, 163: 35–43. doi: 10.1016/j.csr.2018.04.014 [74] Wei Guangshan, Li Mingcong, Li Fenge, et al. 2016. Distinct distribution patterns of prokaryotes between sediment and water in the Yellow River Estuary. Applied Microbiology and Biotechnology, 100(22): 9683–9697. doi: 10.1007/s00253-016-7802-3 [75] Wei Yongjun, Zhang Lei, Zhou Zhihua, et al. 2018. Diversity of gene clusters for polyketide and nonribosomal peptide biosynthesis revealed by metagenomic analysis of the Yellow Sea sediment. Frontiers in Microbiology, 9: 295. doi: 10.3389/fmicb.2018.00295 [76] Wu Ying, Zhu Kun, Zhang Jing, et al. 2019. Distribution and degradation of terrestrial organic matter in the sediments of peat-draining rivers, Sarawak, Malaysian Borneo. Biogeosciences, 16(22): 4517–4533. doi: 10.5194/bg-16-4517-2019 [77] Ye Qi, Wu Ying, Zhu Zhuoyi, et al. 2016. Bacterial diversity in the surface sediments of the hypoxic zone near the Changjiang Estuary and in the East China Sea. MicrobiologyOpen, 5(2): 323–339. doi: 10.1002/mbo3.330 [78] Yu Y, Lee C, Kim J, et al. 2005. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnology and Bioengineering, 89(6): 670–679. doi: 10.1002/bit.20347 [79] Yu Yue, Wang Hui, Liu Jian, et al. 2012. Shifts in microbial community function and structure along the successional gradient of coastal wetlands in Yellow River Estuary. European Journal of Soil Biology, 49: 12–21. doi: 10.1016/j.ejsobi.2011.08.006 [80] Zhang Jing, Du Ya’nan, Zhang Guosen, et al. 2021. Increases in the seaward river flux of nutrients driven by human migration and land-use changes in the tide-influenced delta. Science of the Total Environment, 761: 144501. doi: 10.1016/j.scitotenv.2020.144501 [81] Zhang Xiaohui, Müller M, Jiang Shan, et al. 2020a. Distribution and flux of dissolved iron in the peatland-draining rivers and estuaries of Sarawak, Malaysian Borneo. Biogeosciences, 17(7): 1805–1819. doi: 10.5194/bg-17-1805-2020 [82] Zhang Chunfang, You Shaohong, Dang Hongyue, et al. 2019. Redox characterization of humins in sediments from the Yangtze Estuary to the East China Sea and their effects on microbial redox reactions. Journal of Soils and Sediments, 19(5): 2594–2603. doi: 10.1007/s11368-018-02235-w [83] Zhang Zhaoru, Zhou Meng, Zhong Yisen, et al. 2020b. Spatial variations of phytoplankton biomass controlled by river plume dynamics over the lower Changjiang Estuary and adjacent shelf based on high-resolution observations. Frontiers in Marine Science, 7: 587539. doi: 10.3389/fmars.2020.587539 [84] Zhu Q, van Prooijen B C, Wang Z B, et al. 2017. Bed-level changes on intertidal wetland in response to waves and tides: A case study from the Yangtze River Delta. Marine Geology, 385: 160–172. doi: 10.1016/j.margeo.2017.01.003 -
 22-01 Wei Yongjun Supplementary information 上传-pdf.pdf
22-01 Wei Yongjun Supplementary information 上传-pdf.pdf
-





 下载:
下载: