Diversity of protease-producing bacteria in the Bohai Bay sediment and their extracellular enzymatic properties
-
Abstract: Protease-producing bacteria play key roles in the degradation of organic nitrogen materials in marine sediments. However, their diversity, production of proteases and other extracellular enzymes, even in situ ecological functions remain largely unknown. In this study, we investigated the diversity of cultivable extracellular protease-producing bacteria in the sediments of the Bohai Bay. A total of 109 bacterial isolates were obtained from the sediments of 7 stations. The abundance of cultivable protease-producing bacteria was about 104 CFU/g of sediment in all the samples. Phylogenetic analysis based on 16S rRNA gene sequences classified all the isolates into 14 genera from phyla Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria, with Pseudoalteromonas (63/109, 57.8%), Bacillus (9/109, 8.2%), Sulfitobacter (8/109, 7.3%) and Salegentibacter (6/109, 5.5%) as the dominant taxa. Enzymatic inhibition tests indicated that all the tested isolates produced serine and/or metalloprotease, with only a small proportion producing cysteine and/or aspartic proteases. Several extracellular enzyme activities, including alginase, lipase, amylase and cellulose, and nitrate reduction were also detected for strains with higher protease activities. According the results, the protease-producing bacteria could also be participate in many biogeochemical processes in marine sediments. Our study broadened understanding and knowledge on the potential ecological functions of protease-producing bacteria in marine sediments.
-
Key words:
- protease-producing bacteria /
- diversity /
- inhibition test /
- multi-extracellular enzymes
-
1. Introduction
Polymeric and particulate organic matters carry abundant organic nitrogen (OrgN), which are considered the main nitrogen sources in marine sediment environments (Thamdrup and Dalsgaard, 2008). In the nitrogen cycle, the OrgN are usually decomposed into dissolved OrgN, followed by a series of ammonification, nitrification and denitrification processes that are carried out by marine microorganisms (Hunter et al., 2006; Thamdrup and Dalsgaard, 2008). Proteins are the main building blocks of organismal tissues, and protease producing bacteria are known as the main degraders of organic nitrogen in the marine environment (Zhang et al., 2015; Zhou et al., 2009). These bacterial groups secrete extracellular proteases that degrade proteins into peptides and amino acids, which can be easily taken up by other bacteria for subsequent metabolism and processing (Zhao et al., 2012). Therefore, protease-producing bacteria play important roles in the biogeochemical cycles of marine environments, especially in the sediment ecosystems. However, the diversity of these bacteria and their extracellular enzymes remain largely unknown.
Protease producing bacteria are widely spread in marine sediments. For example, 30 out of the 98 strains isolated from Southern Okinawa Trough possessed protease-producing abilities, distributed in the genera Bacillus, Cobetia, Halomonas, Pseudomonas, Psychrobacter, Myroides, Planococcus, Sporosarcina and Wangia (Dang et al., 2009). By using selective media, diverse protease producing bacteria were isolated from the sub-Antarctic sediment (Olivera et al., 2007), the deep South China Sea sediment (Zhou et al., 2009), coastal sediments of King George Island, Antractica (Zhou et al., 2013), enclosed the Jiaozhou Bay (Zhang et al., 2015) and the Laizhou Bay (Li et al., 2017). In these studies, the protease producing bacteria were classified under the genera Alcanivorax, Alteromonas, Burkholderia, Caulobacter, Celeribacter, Halomonas, Hyphomonas, Idiomarina, Marinobacter, Microbulbifer, Photobacterium, Pseudoalteromonas, Pseudomonas, Psychrobacter, Rheinheimera, Ruegeria, Shewanella, Sphingopyxis, Sulfitobacter and Vibrio of the phylum Proteobacteria; Bacillus, Exiguobacterium, Halobacillus, Jeotgalibacillus, Oceanobacillus and Planococcus of Firmicutes; Aequorivita, Asinibacterium, Flavobacterium, Formosa, Gillisia, Lacinutrix, Psychroserpens, Salegentibacter, Olleya and Zobellia of Bacteroidetes; Arthrobacter, Janibacter, Leifsonia, Microbacterium, Micrococcus, Nocardioides, Nocardiopsis and Streptomyces of Actinobacteria, dominated by Alteromonas, Bacillus, Flavobacterium, Lacinutrix, Photobacterium, Pseudoalteromonas and Vibrio. And analyses of hydrolytic abilities towards different protein substrates and inhibition tests indicated that all of these bacteria could decompose casein and/or gelatin and produce serine and/or metalloproteases (Li et al., 2017; Olivera et al., 2007; Zhang et al., 2015; Zhou et al., 2009, 2013).
Although the culture independent approaches has been applied to detecting microbial resource reserves including the uncultivable taxa, isolation remains a necessary approach to obtain novel microbes especially for screening specific functional isolates with biotechnological application potentials (He et al., 2016; Sfanos et al., 2005). For example, recent studies on protease-producing bacteria using culturing method still obtained new groups of bacteria possessing higher protease producing abilities than previous studies (Li et al., 2017).
The Bohai Bay, located in the northern coast of China, covers a total area of 14 700 km2, corresponding to 20% of the total area of the Bohai Sea. It is the largest semi-enclosed shallow water basin in the western region of the Bohai Sea. It is an important spawning and traditional fishing grounds, and an aquaculture zone for economically important fish, shrimp and crab species (Fu et al., 2016; Hu et al., 2010; Sun et al., 2011; Mu et al., 2017). In addition, the Bohai Bay costal area is one of the three most densely populated and developed zones in China. Large volumes of sewage water containing heavy metal and organic pollutants being dumped into it resulted to it becoming the most heavily exploited and polluted marine areas in China. Therefore, the deterioration of water quality accompanied by algal blooms, have occurred as manifestations of severe environmental problems in this sea (Feng et al., 2011; Hu et al., 2010; Mu et al., 2017). With the increasing problems on water pollution, the microbial community in this aquatic ecosystem, especially the bacteria have abilities to degrade organic materials, such as protein, alginate and some pollutant chemical compound, are also expected to vary. However, the diversity of protease-producing bacteria in this ecosystem and the characteristics of the proteases they produce remain little understood. Understanding of the proteases and other extracellular enzymes produced by these bacteria could not only facilitate the exploration of the other novel enzyme-producing bacteria for biotechnological or industrial applications but would also help elucidate in situ biogeochemical processes mediated by these microorganisms. Aiming to uncover the diversity of cultivable protease-producing bacterial community in the Bohai Bay, we isolated the proteolytic bacteria and characterized their extracellular proteases using hydrolytic activity towards different protein substrates. We also tested factors that could inhibit their activity. Lastly, several other extracellular enzymes production and nitrate reduction abilities were also tested.
2. Materials and methods
2.1 Ocean sediment collection and physicochemical character determination
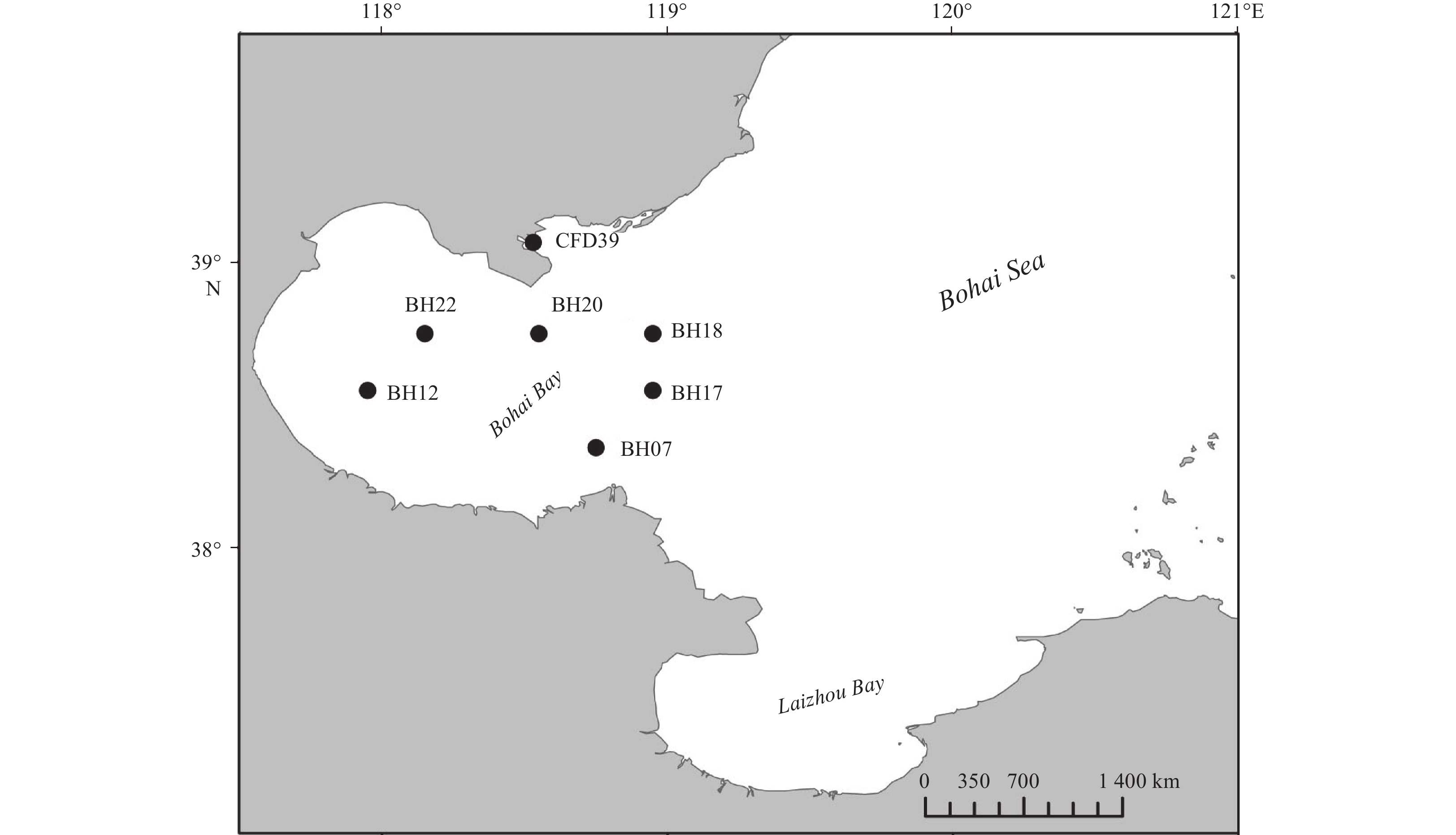
A total of 7 sediment samples were collected from the Bohai Bay (GPS: 38.35°–39.07°N and 117.95°–118.95°E, Fig. 1 and Table 1) in April 2014 using a 0.05 m2 stainless steel Gray O’Hara box corner as previously described (Li et al., 2017). For each sampling site, triplicate surface sediment soil samples (0–5 cm depth) were collected using sterilized 60 mL syringes (without Luer end) and then transferred to airtight sterile plastic bags and stored at 4°C until analysis in the laboratory (Li et al., 2017; Zhou et al., 2009).
Table 1. Characteristics of the sampling stations and the distribution of different genera in these stationsProperties Station BH07 BH12 BH17 BH18 BH20 BH22 CFD39 GPS 38.35°N, 118.75°E 38.55°N, 117.95°E 38.55°N, 118.95°E 38.75°N, 118.95°E 38.75°N, 118.55°E 38.75°N, 118.15°E 39.07°N, 118.53°E Characteristics for sediment samples Depth/m 16.14 9.66 22.65 25.57 23.78 17.27 12.19 Temperature/°C 24.97 27.11 20.36 19.32 23.27 25.47 26.14 pH 8.11 8.08 7.91 7.90 8.01 8.18 8.08 DO 4.02 4.17 2.20 2.28 3.50 5.11 4.86 Sal 30.30 30.02 30.31 30.45 30.51 30.20 30.63 OrgC/% 0.42 0.51 0.55 0.60 0.54 0.57 0.87 OrgN/% 0.06 0.08 0.09 0.08 0.09 0.10 0.11 C/N1) 7.00 6.38 6.11 7.5 6.00 5.70 7.91 Genera distribution Proteobacteria Pseudoalteromonas 3 8 15 6 16 7 8 Shewanella 3 1 Marinobacter 1 3 Sulfitobacter 4 4 Celeribacter 1 1 1 Albirhodobacter 1 1 Firmicutes Bacillus 7 1 1 Halobacillus 1 1 1 Fictibacillus 1 Actinobacteria Micrococcus 1 1 Citricoccus 1 Brachybacterium 1 Bacteroidetes Arenibacter 2 Salegentibacter 2 2 2 Total strain number (109) Diversity index Shannon–Wiener (H′) 1.58 1.22 1.66 1.01 0.34 0.94 0.64 Simpson (D) 0.72 0.61 0.71 0.61 0.20 0.48 0.34 Pielou (J) 0.81 0.76 0.76 0.92 0.50 0.68 0.58 Note: 1) C/N is the abbreviation of OrgC/OrgN. For physicochemical analyses, sediment samples were collected with the same procedure and stored in sterilized plastic bags at –20°C and in the dark during the cruise and were transferred to –80°C immediately after arrival at the laboratory. Sediment surface temperature and salinity were detected using Conductivity, Temperature, and Depth (CTD) Data Acquisition System (MIRAI MR99), and pH was determined in situ using a pH meter. Organic carbon (OrgC) and organic nitrogen (OrgN) concentrations were quantified using a PE 2400 series II CHNS/O analyzer (Perkin Elmer, USA).
2.2 Isolation of protease-producing bacteria from sediment Ssamples
Protease-producing bacteria were isolated from sediment samples with a selective medium following previous studies (Li et al., 2017; Zhou et al., 2009). In brief, three samples from the same station were mixed together in equal weight and 1 g of the freshly mixed sediment sample was serially diluted to 10–6 with sterilized artificial sea water. Aliquots of 100 μL of each dilution (10–1–10–6) were separately spread on the screening plates composed of 2 g yeast extract, 3 g casein, 5 g gelatin, 15 g agar powder in 1 L artificial seawater at pH 7.0 (Zhou et al., 2009). All of the inoculated plates were incubated at 25°C until colonies with clear hydrolysis zones were detected. Colonies with different morphological characteristics (e.g., colony color, size and surface polysaccharides) were selected and further purified by streaking repeatedly on the same plate until pure colonies were observed. All pure cultures were preserved at –80°C in 20% (v/v) glycerol.
2.3 Phylogenetic analyses
Each pure isolate was incubated in marine broth 2216 medium (Li et al., 2017) at 25°C with shaking of 200 r/min. Their biomasses were separately harvested by centrifugation and the genomic DNA was extracted using a TIANGEN genomic DNA extraction kit for bacteria (TIANGEN, China). Both DNA quality and quantity were estimated in Nano OD2000C (Thermo). The 16S rRNA gene of all the isolates were amplified and sequenced using the universal primer pairs 27F/1492R at PCR conditions described in Engel et al. (2004). Samples were sent to Beijing AuGCT DNA-SYN Biotechnology Co., Ltd for sequencing using the Sanger method (Sanger et al., 1977). The quality of sequences were manually checked in BioEdit software version 7.0 and searched for their most similar sequences against the NCBI GenBank using the BLASTn approach and also with EzTaxon server (http://www.ezbiocloud.net/). Most similar sequences were downloaded and a phylogenetic tree was reconstructed based on 16S rRNA gene sequences using the neighbor-joining method (Saitou and Nei, 1987) with Kimura’s two-parameter model in MEGA version 6.0 (Tamura et al., 2013). The topology of the phylogenetic tree was evaluated by bootstrap resampling method with 1 000 replicates (Felsenstein, 1985). All sequences obtained in this study were deposited in GenBank database (Table S1).
Due to highly conserved 16S rRNA gene sequences, which limits the resolution in delineating at the species level (Li et al., 2017), all isolates were only classified at the genera level. Alpha diversity of the community for each sample was calculated at the genus level using three indices, namely, Shannon–Wiener index (H′) that shows the genera richness at a sampling site, the Simpson index (D) that explains the genera dominance, and the Pielou index (J) that indicates species evenness in a community (Hill et al., 2003). The biodiversity indices were calculated using the Vegan package (version 1.17–4) performed in the R environment (version 3.3.2; http://www.r-project.org/) (R Core Team, 2014).
2.4 Inhibition ratio (%) of different inhibitors on the protease activity
All protease-producing bacteria isolated in this study were then cultivated in the liquid screening medium (screening media without agar, 100 mL), and were incubated at 25°C with shaking of 200 r/min for about 72 h. Then, the liquid cultures were centrifuged at 12 000 r/min for 2 min to collect the supernatant, which were subsequently used to measure the protease activity (Chen et al., 2003). The inhibition tests were performed as described previously (Li et al., 2017). In brief, 1 mL of the supernatants were diluted with 50 mmol/L Tris-HCl containing 2.0% (w/v) casein (pH 8.0), which was pre-incubated with 1.0 mmol/L phenylmethylsulfonyl fluoride (PMSF, Sigma; serine protease inhibitor), 1.0 mmol/L 1, 10-phenanthroline (OP, Sigma; metalloprotease inhibitor), 0.1 mmol/L M E-64 (Merk; cysteine protease inhibitor) and 0.1 mmol/L pepstatin A (P-A, Sigma; aspartic protease inhibitor) at 25°C for 20 min. After incubation, the reaction was stopped by adding 2 mL of 0.4 mol/L trichloroacetic acid. Finally, the supernatant was neutralized with 0.4 mol/L sodium carbonate, incubated with Folin–Ciocalteu’s reagent solution (Sigma) and the protease activity was separately measured at 660 nm for each sample (Chen et al., 2003; Li et al., 2017; Zhang et al., 2015). One unit of enzyme activity was defined as the formation of 1 μmol tyrosine in 1 min (Li et al., 2017). The protease activity of the sample without any inhibitor was used as the positive control. Then, the inhibition ratio (I, %) was calculated as I = (Ac – As) × 100/Ac, where Ac was the activity in the positive control, and As that of the sample (Li et al., 2017; Zhang et al., 2015; Zhou et al., 2009). Finally, only 50 out of 109 isolates produced enough enzymes useful in determining the inhibition ratio.
2.5 Hydrolytic activities of extracellular enzymes against casein, gelatin and elastin
A basic medium containing 0.2% (w/v) yeast extract, 0.5% (w/v) peptone, 1.5% (w/v) agar was dissolved in artificial seawater at pH 8.0, supplemented with 0.5% (w/v) each of casein, gelatin or elastin to prepare the plates (Zhou et al., 2009). All 109 isolates from sediment samples were inoculated with sterilized toothpick on the media plates and incubated at 25°C for 3 d. Then, the diameters of the colony and the hydrolyzed zone were measured for each isolate, and a ratio of the hydrolytic zone diameter vs. the colony diameter (hydrolytic zone/colony, H/C) was calculated as a proxy for enzyme activity against each substrate (Li et al., 2017; Zhou et al., 2009).
2.6 Production of other extracellular enzymes and nitrate reduction test
The 50 representative strains, which produced enough extracellular protease to conduct inhibition tests were selected to determine other extracellular enzymes produced. Activities of alginase, lipase, amylase and cellulase were analyzed as previously described (Kitamikado et al., 1990; Hansen and Sørheim, 1991; Yadav et al., 2015), and using H/C ratio (hydrolytic zone/colony, H/C) as a proxy for enzyme activity for each substrate. Enzyme substrates were obtained from Sigma Company. Nitrate reduction assay was performed according to Hansen and Sørheim (1991), in brief, the tested isolates were inoculated for 5 d in liquid media, which contained 0.5 g MgSO4, 0.5 g K2HPO4, 1 g KNO3, 20 g sucrose, 0.5 g NaCl in 1 L of artificial sea water. Then the test was read by overlaying the wells with a mixture of sulphanilic acid and α-naphthylamine. Positive isolates produced bright red discoloration within 2–3 min, while negative isolates did not change the color and turned red color after addition of powdered Zn.
3. Results
3.1 Sampling site and sample characteristics
The water depths ranged from 9.66 m in Station BH12 to 25.57 m in Station BH18 (Fig. 1 and Table 1). All sediment samples showed slight alkalinity with pH varying from 7.90 in BH18 and 8.18 in BH22. The OrgC and of OrgN concentrations in the sediments ranged from 0.42% to 0.87% and 0.06% to 0.11%, respectively, with BH07 and CFD39 presenting the lowest and the highest values for both OrgC and OrgN. Furthermore, the highest C/N ratio (7.91) was also observed in Station CFD39 and the lowest (5.70) in Station BH22.
3.2 Isolation and quantification of protease-producing bacteria
Colonies with different colors, morphologies and sizes appeared on the screening plates inoculated with 10–2–10–4 dilutions after incubating at 25°C for 1–5 d, with the richness of about 104 CFU (colony forming unit) per gram of sediment soil samples. Furthermore, nearly 80% of the colonies exhibited proteolytic zones. No obvious correlation between the richness of protease-producing bacteria and organic material was observed. Finally, 109 proteolytic colonies were purified and selected for subsequent analysis.
3.3 Diversity of the protease-producing bacteria Isolated from sediments
High quality nearly full-length 16S rRNA gene sequences (> 1 400 bp) were generated from all 109 isolates. Phylogenetic analysis classified them into 14 genera in the phyla Proteobacteria, Firmicutes, Actinobacteria and Bacteroidetes (Fig. 2, Table 1 and Table S2).
 Figure 2. Phylogenetic tree of the protease-producing bacteria isolated from the Bohai Bay based on 16S rRNA gene sequences. Taxa and GenBank accession numbers in boldface were generated in this study. The tree was constructed by neighbor-joining method using MEGA version 6.0. Only bootstrap values greater than 50% are presented in the nodes. The scale bar represents 2% nucleotide substitution.
Figure 2. Phylogenetic tree of the protease-producing bacteria isolated from the Bohai Bay based on 16S rRNA gene sequences. Taxa and GenBank accession numbers in boldface were generated in this study. The tree was constructed by neighbor-joining method using MEGA version 6.0. Only bootstrap values greater than 50% are presented in the nodes. The scale bar represents 2% nucleotide substitution.Two isolates from Station BH17 belonged to Arenibacter and 6 isolates from BH17, 18 and 20 isolates belonged to Salegentibacter in the phylum of Bacteroidetes. Two isolates from BH22 and BH 17 were affiliated with Citricoccus and Brachybacterium, respectively and another two belonged to Micrococcus in the phylum Actinobacteria. The remaining isolates were classified into 9 genera in Phyla Proteobacteria (84/109, 77.06%) and Firmicutes (11.93%), including Pseudoalteromonas, Shewanella and Marinobacter within the class Gammaproteobacteria; Sulfitobacter, Celeribacter and Albirhodobacter within the class Alphaproteobacteria in Proteobacteria; Bacillus, Halobacillus and Fictibacillus within the class Bacilli in Firmicutes.
Pseudoalteromonas (63/109, 57.8%), Bacillus (9/109, 8.3%), Sulfitobacter (8/109, 7.3%) and Salegentibacter (6/109, 5.5%) were the dominant genera, while Fictibacillus, Citricoccus and Brachybacterium were represented only by a single isolate for each. The most abundant genus Pseudoalteromonas was isolated in all stations and dominated in nearly all stations except BH07, which was dominated by Bacillus. Furthermore, a total of 9 genera protease-producing bacteria were isolated from BH17, which showed higher diversity than those isolated from other sampling sites. In contrast, only two genera were identified in Station BH20, representing the least diverse community among the 7 sediment samples.
Twenty-eight of the 63 Pseudoalteromonas isolates from Stations BH17, BH18 and BH20 formed a distinct cluster (Branch 1) in the constructed phylogenetic tree (Fig. 2, also see Table 1 and Fig. S1) that shared 99.7%–100% of 16S rRNA gene similarities. Most similar references included Pseudoalteromonas atlantica NBRC 103033T and Pseudoalteromonas hodoensis H7T, which were isolated from seaweeds in Japan (Akagawa-Matsushita et al., 1993) and coastal sea water in Korea, respectively (Chi et al., 2014), all of which possess the ability to decompose algal polysaccharides. Meanwhile, 25 isolates from 6 stations formed Branch 2 (Fig. 2 and Fig. S2) with 99.6%–100% similarities, clustering closely with Pseudoalteromonas spiralis Te-2-2T and P. haloplanktis NBRC 102225T. A total of 5 isolates (70002, 70006, 70008, 70024 and 70053) were identical to Pseudoalteromonas distincta KMM638T. Strains 70038 and 70039 sequences were highly similar (99.7%) with 70040, which in turn were 99.5%–99.8% similar to P. distincta KMM638T. On the other hand, Isolates 70023 and 70056 were 99.9% similar with each other, and closest to Pseudoalteromonas mariniglutinosa KMM 3635T (98.8%–99.9%). Shewanella isolates (70058, 70059, 70060 and 70319) shared 99.9%–100% similarities to Shewanella algidipiscicola NBRC 102032T, while 4 Marinobacter isolates (70052, 70061, 70434 and 70435) shared 99.7%–100% similarities among them and most similar to Marinobacter lipolyticus SM-19T (99.6%–99.9%). Strains 70046 and 70069 shared 99.9% similarities. Three Celeribacter strains 70047, 70067 and 70078 were most similar with Celeribacter baekdonensis L-6T, and the 8 Sulfitobacter were most related to Sulfitobacter pontiacus ChLG-10T with 99.9% sequence similarities. Strains 70025 and 70027 shared 99.9% similarities and were most related to Micrococcus yunnanensis YIM 65004T. Strain 70028 was closely related to Citricoccus parietis 02-Je-010T with 99.0% sequence similarity. Strain 70026 was most related to Brachybacterium alimentarium CNRZ 925T with 99.0% similarity. Isolate 70014 was closely related to Fictibacillus arsenicus Con a/3T with 98.9% sequence similarity. Isolates 70057 and 70075 shared identical sequences and were most related to Halobacillus litoralis SL-4T with 99.9% similarities, while 70074 was most related to Halobacillus faecis NBRC 103569T with 99.9% similarity. Nine Bacillus isolates (Fig. 2) showed high similarities (>99.0%) in 16S rRNA gene sequences with reference strains for different defined species, including Bacillus hemicentroti JSM 076093T, Bacillus frigoritolerans DSM 8801T and Bacillus altitudinis 41KF2bT etc.. The 70068 and 70414 shared 99.2% similarity and they were closely related to Arenibacter echinorum KMM 6032T (99.9%) and A. hampyeongensis HP12T (99.5%), respectively. Isolates 70304, 70390, 70401, 70410 and 70416 shared high similarity with 70301 (99.9% similarities), and all were closely related to Salegentibacter salinarum ISL-4T (99.6% sequence similarities).
Shannon-Weiner (H′) index was highest in Station BH17 (1.66), followed by Stations BH07 (1.58) and BH12 (1.22) (Table 1). The lowest value (0.34) was detected in Station BH20 where only two genera were found. The highest Simpson index (D) value (0.72) was found in Station BH07, followed by Stations BH17 (0.71), BH12 (0.61) and BH18 (0.61). The lowest value was observed in Station BH20 (0.20). The Pielou (J) value ranged from 0.50 in Station BH20 to 0.92 in Station BH18.
3.4 Diversity of the extracellular proteases produced by the bacteria
The diversity of proteases produced by the bacteria was investigated through inhibitor analyses and their hydrolytic abilities against different types of protein substrates (Table 2 and Table S3). Out of the 109 isolates, only 50 produced enough proteases for enzymatic inhibition analysis. PMSF inhibited the activities of all the 50 sub-selected isolates with varying levels from 9.42% to 99.23%, indicating that all of the isolates produced serine proteases in different proportions. Furthermore, the enzyme activities of 6 isolates were inhibited (>90%) by PMSF, demonstrating that they mainly or only produced serine proteases. The enzyme activities of 40 isolates were inhibited by OP, among which 23 isolates were efficiently inhibited (20.35%–53.84%) and 18 isolates were slightly inhibited (1.31%–18.48%), while no inhibitory effect on 10 isolates was observed, suggesting that most of the isolates produced metalloproteases. Meanwhile, 40 out of 50 (accounting 80%) isolates were inhibited by both PMSF and OP at different levels indicating that most isolates in this study produced both serine proteases and metalloproteases. There were only 14 isolates that were inhibited by E-64 and 8 isolates by PA, but the inhibition ratios were less than 10%, illustrating that these isolates produced very low amounts of cysteine or aspartic protease. The results suggest that nearly all extracellular proteases produced by the tested bacteria belonged to serine protease and/or metalloproteases.
Table 2. Summary of the inhibition test and the extracellular enzyme production analyses of the tested strains isolated from the Bohai BayGenera Strain Inhibition ratio1) (I)/% H/C ratio2) Nitrate PMSF O-P E-64 P-A Casein Gelatin Elastin Amylase Cellulase Alginase Tween 80 Pseudoalteromonas 70004 43.64 22.87 0 0 1.77 3.00 1.27 0 0 0 0 – 70006 60.97 0 3.35 0 2.78 2.92 1.40 4.00 2.18 3.26 6.31 + 70032 80.75 46.83 0 0 1.54 3.10 0 0 0 0 1.70 70038 16.89 0 0 0 2.33 4.50 0 0 0 0 1.61 – 70039 43.83 0 0 0 2.50 4.71 0 0 3.68 1.11 3.27 70048 48.41 16.53 0 0 2.78 5.00 0 0 0 0 4.52 + 70051 71.35 45.19 0 2.99 3.25 2.67 1.86 0 0 0 2.59 – 70053 76.43 29.47 0 0 2.25 5.00 0 0 0 1.49 5.40 – 70056 80.05 0 0 0 2.78 4.14 0 0 0 3.60 0 – 70305 46.37 0 0 0 3.42 1.40 2.21 1.59 0 1.20 3.17 – 70306 49.18 34.79 4.11 0 2.87 3.22 1.58 1.76 1.08 1.25 1.51 + 70307 81.10 44.84 0 0 2.94 2.56 1.92 3.23 0 1.33 3.36 – 70308 99.23 48.20 5.80 0 2.75 2.87 1.58 2.07 0 1.16 3.83 + 70320 52.13 5.81 0 0 2.31 1.46 1.55 1.49 1.22 1.22 1.51 – 70325 39.57 6.21 0 0 3.14 1.27 1.70 1.98 0 1.44 2.99 + 70326 81.42 34.55 0 0 3.19 1.14 2.10 2.19 0 1.96 1.70 – 70333 29.63 6.34 0 0 2.62 1.20 1.55 1.64 0 1.20 2.79 – 70339 90.13 31.28 4.48 4.48 2.00 0 1.50 1.61 4.11 1.23 3.46 + 70342 85.92 3.20 0 0 2.18 1.45 1.58 0 0 0 0 – 70344 70.04 53.84 7.04 0 2.38 1.42 1.25 1.34 0 1.28 2.96 – 70354 51.04 20.35 4.25 0 3.43 2.85 1.73 1.26 0 1.32 1.33 – 70356 68.40 10.60 0 7.72 3.18 1.57 2.09 1.75 0 1.31 1.19 – 70359 77.04 42.95 0 3.74 3.42 1.80 1.60 1.83 0 1.86 2.64 + 70361 50.74 6.17 3.60 0 3.00 2.36 1.77 1.35 0 2.12 2.83 – 70365 92.29 27.77 0 0 3.21 3.29 2.50 1.34 0 2.18 0 + 70369 57.37 34.39 0 0 2.44 2.08 1.50 1.76 1.41 2.37 1.18 – 70373 95.19 44.79 0 3.12 3.29 3.17 1.50 2.97 1.12 3.36 2.73 + 70374 76.82 37.19 0 0 3.13 2.27 1.36 2.36 0 6.55 2.78 – 70375 61.08 0 2.23 0 2.93 2.88 2.11 2.89 0 4.63 0 – 70376 15.39 5.92 0 0 3.00 2.75 1.33 0 0 2.16 0 – 70379 42.46 51.17 3.70 0 2.80 2.64 1.80 1.27 0 3.29 2.79 – 70380 91.52 51.24 1.28 4.57 2.82 3.15 1.64 0 1.59 2.10 0 – 70413 99.18 32.76 0 0 2.29 1.27 1.33 1.40 0 2.62 2.71 – Bacillus 70013 63.69 8.67 0 0 1.80 3.67 0 0 0 8.74 0 – 70035 9.42 0 0 0 1.40 6.50 0 0 0 0 0 + 70037 19.80 3.89 0 0 2.14 7.00 0 0 0 2.96 2.04 – 70049 92.58 44.74 0 3.09 4.80 5.42 1.32 0 0 0 0 – 70055 31.98 18.38 7.45 7.63 1.36 3.86 0 0 0 0 0 – Fictibacillus 70014 43.21 1.84 0 0 1.67 5.00 0 0 0 2.52 0 – Micrococcus 70025 68.60 24.30 7.83 0 4.00 5.17 0 0 0 0 0 – 70027 61.26 17.12 9.01 0 3.80 4.14 0 0 0 1.88 0 – Halobacillus 70074 38.71 0 0 0 3.57 3.33 2.29 0 0 2.95 0 – 70075 63.57 15.94 0 0 2.00 4.00 0 0 1.37 0 0 – Salegentibacter 70304 35.37 6.19 7.24 0 4.60 1.29 5.00 0 0 2.63 0 + 70390 84.24 0 0 0 8.33 7.83 5.40 0 0 3.41 0 + 70400 42.39 0 0 0 5.00 4.60 3.57 0 0 2.00 0 – 70401 46.26 8.49 0 0 6.75 6.00 5.00 0 0 2.62 0 + 70416 20.22 1.31 0 0 6.00 3.00 5.50 0 0 2.11 0 + Marinobacter 70434 41.70 37.85 0 0 2.09 0 0 3.54 0 5.27 2.40 + 70435 38.74 35.58 0 0 1.28 3.19 0 3.82 0 7.84 2.40 + Note: 1) Inhibition ratio (I, %) was calculated by using control activity minus the relative activity of a sample with an inhibitor and the activity of a sample without any inhibitor was taken as a control. 2) H/C ratio is the ratio of the hydrolytic zone diameter versus the colony diameter of a colony on the plate. PMSF, phenylmethylsulfonyl fluoride; OP, 1, 10-phenanthroline; P-A, pepstatin A. The diversity of extracellular proteases produced by the 109 isolates from the Bohai Bay was also investigated through the hydrolytic abilities (H/C ratio) against different protein substrates (Table 2 and Table S3). Most of the isolates formed apparent hydrolytic zones on the plates containing casein or gelatin, except 70391, 70424 and 70432 that could not hydrolyze casein, and 70335, 70339, 70417, 70433 and 70434 that were not capable of hydrolyzing gelatin. The Salegentibacter isolates 70390, 70400, 70401 and 70416 showed high caseinolytic activity with H/C ratio greater than 5.0, with 70390 exhibiting the highest H/C ratio of 8.33. Further, Salegentibacter (70390 and 70401), Bacillus (70035, 70037 and 70049), Fictibacillus (70014), Micrococcus (70025) and Pseudoalteromonas (70048, 70053) presented strong gelatinolytic activity with H/C ratio greater than 5.0, but it was the Salegentibacter isolate 70390 that had the highest H/C ratio of 7.83. Only 46 (42.2%) isolates exhibited ability to hydrolyze elastin, with the Salegentibacter isolates 70304, 70390, 70401 and 70416 being the most active, and the latter having the highest activity ratio of 5.50. All the elastinolytic isolates also showed caseinolytic and gelatinolytic activities except for 70339 that could not hydrolyze gelatin. Salegentibacter isolates 70390 and 70401 could hydrolyze all the three protein substrates with an H/C ratio greater than 5. Furthermore, varied hydrolytic abilities were observed among the isolates even for the isolates having identical 16S rRNA gene sequences.
3.5 Other extracellular enzyme production and nitrate reduction test
All 50 isolates showing inhibition effects were further evaluated for their hydrolytic abilities (H/C ratio) against the substrates alginate sodium, Tween 80, soluble starch and cellulose. Around 39 (78%) out of the 50 tested isolates produced alginase, with 10 isolates exhibiting H/C ratio greater than 3.0, with Isolates 70013, 70374, 70434 and 70435 presenting H/C ratios greater than 5.0. All Salegentibacter isolates degraded alginate sodium efficiently. On the other hand, 29 isolates (58%) formed precipitates on the Tween 80 plates, where 8 isolates showed H/C ratios greater than 3.0, and 70006 and 70053 had greater than 5.0. Meanwhile, 24 isolates (48%) formed hydrolytic zones on soluble starch plate, in which 70006, 70307, 70434 and 70435 presented H/C ratios greater than 3.0. Only 9 isolates (18%) possessed the abilities to hydrolyze cellulose and Isolates 70039 and 70339 exhibited ratios greater than 3.0. Thus, most tested isolates produced alginase, nearly half of them produced amylase and lipase, and about 20% of the isolates produced cellulases. Lastly, 16 isolates (32%) tested positive on nitrate reduction test, including 4 of the 5 tested Salegentibacter isolates.
4. Discussion
In the present study, sediment samples in the seven sampling sites at the Bohai Bay had relatively lower C/N ratios (5.70 to 6.91, with average 6.66) and richness of cultivable protease-producing bacteria (104 CFU/g of sediment) compared with the sediment samples from the other two semi-enclosed bays, Jiaozhou Bay and Laizhou Bay, in China (Li et al., 2017; Zhang et al., 2015). The high evenness of the protease-producing bacteria in the sediment samples indicates a relatively stable population size. No significant correlations were observed between bacterial diversity or composition and OrgC, OrgN, and their ratios (OrgC/OrgN), consistent with previous reports (Li et al., 2017; Zhang et al., 2015; Zhou et al., 2009, 2013). Station CFD39 near the newly reclaimed area characterized by more anthropogenic disturbance, was the most polluted among the seven stations. Despite this, the bacterial community structure in this station was not significantly different from the other six stations.
Bacterial diversity has been rarely investigated in the Bohai Bay. A recent investigation based on culture-independent (RFLP of 16S rRNA gene) method revealed the diverse communities in sediments of the Bohai Bay, belonging to at least six defined phyla and other unclassified phyla with Proteobacteria as the dominant phylum and Gammaproteobacteria as the dominant class (Sun et al., 2011). Similar observations were seen in our study, where both Proteobacteria and Gammaproteobacteria dominated the phylum and class groupings of the communities, respectively. Furthermore, the dominance of Pseudoalteromonas (63 isolates, 88.7% of the Gammaproteobacteria isolates) in all the seven samples was consistent with the results of previous studies in the South China Sea and Laizhou Bay sediments (Li et al., 2017; Zhou et al., 2009). Firmicutes was the second most abundant phylum, dominated by Bacillus (9 isolates, 8.3% of all the isolates and 61.5% of the Firmicutes isolates), which again was consistent with previous reports in the sediments of coastal sub-Antarctic, Jiaozhou Bay and Laizhou Bay (Li et al., 2017; Zhang et al., 2015; Zhou et al., 2009). The Bohai Bay is a traditional aquaculture zone and a spawning ground for many marine animals (Sun et al., 2011). Bacillus species are known to degrade wastes from aquafarming such that of shrimps, while Pseudoalteromonas are associated with marine animals such as fish (Gamal et al., 2016; Pujalte et al., 2007). In addition, Bacillus and Pseudoalteromonas strains have been suggested to influence the overall community structure through production of inhibitory/antibiotic secondary metabolites (Bowman, 2007; Mondol et al., 2013). Thus, the high proportions of Pseudoalteromonas and Bacillus isolates may be in accordance with their potential ecological functions and antibacterial capacities. These results show that the main proteolytic bacterial functional groups were similar in the marine sediments of the South China Sea, sub-Antarctic, Antractica, Jiaozhou Bay and Laizhou Bay (Olivera et al., 2007; Zhou et al., 2009; Zhang et al., 2015; Li et al., 2017), despite their geographic differences. However, compared with previous studies, this was the first time that bacteria belonging to Albirhodobacter, Fictibacillus, Citricoccus, Brachybacterium and Arenibacter were reported to possess proteolytic abilities. These results show that the marine sediments may be rich resource of novel enzymes for bioengineering.
Similar to previous studies on proteolytic bacteria in the sediments (Li et al., 2017; Zhang et al., 2015; Zhou et al., 2009, 2013), strains detected in this study mainly produced serine and/or metallo-proteases. Furthermore, only a small portion of the isolates exhibited elastin-degrading abilities (Table 2 and Table S3). This could be due to the high molecular size of elastin, making it difficult to degrade (Li et al., 2017). Interestingly, all Salegentibacter isolates in this study exhibited the highest proteolytic activities against all the three substrates (Table 2), which was in accordance with the previous study (Li et al., 2017).
Alginic acid is the major polysaccharide component of brown algae (Anastasakis et al., 2011) and macroalgae, such as Laminaria longissima, which is naturally widely distributed and also cultured in the Bohai Bay (Zhang et al., 2007). Most of the tested 50 isolates produced alginase (Table 2), indicating that they could be capable of degrading dead algae as their carbon sources in the sediments. In marine environments, OrgN, usually in the form of proteins and fats, come from dead animals (Alasalvar et al., 2002). In addition, some algae accumulate large amounts of algal oil (DemiRbas and DemiRbas, 2011). Thus, lipase is also important for survival of bacteria in marine sediments. In this study, more than one half of the tested isolates produced lipase, indicating that these bacteria could degrade fats that rich in the sediments. Starch and cellulose meanwhile are mainly produced by terrestrial plants and some green algae (Itoh, 1990; Smant et al., 1998; Tsekos, 1999; Viola et al., 2001). The detection of amylase production in nearly half of the tested isolates demonstrate the possibility that the sediment bacteria utilize algal materials as their nutrient source. However, the small proportion (18%) of cellulolytic bacteria in the tested isolates in this study suggest that most of the proteolytic bacteria used materials other than cellulose as their C-source.
Protease-producing bacteria are known as the main decomposers of organic nitrogen, converting them into peptides and amino acids (Zhao et al., 2012). However, only a few studies have reported their abilities to reduce nitrate. In this study, nitrate reduction was detected only in 16 out of the 50 tested isolates. Nevertheless, this could indicate their capacity for anaerobic respiration (Putz et al., 2018), which could be an adaptation to the sediment environment, allowing them to be more efficient in mineralization of organic matter. The high proportion of Salegentibacter that possessed proteolytic capacity suggests that they play important roles in the degradation of organic materials in the sediments. Specifically, all the five tested Salegentibacter isolates had the highest proteases and alginase activities, with four of them showing nitrate reduction. The Pseudoalteromonas sp. 70006 possessed the highest amylase and lipase activities, while Pseudoalteromonas sp. 70339 had the highest cellulolytic activity. Our results indicate that proteolytic bacteria play multiple roles in organic matter degradation, and the specificity of their enzyme activities against different substrates (Table 2 and Table S3) allow effective degradation of diverse and complex organic materials, which make them functional exist in multiple marine econiches in the sediments of the Bohai Bay.
In conclusion, this study revealed the community structure of the cultivable protease-producing bacteria in the sediments of the Bohai Bay, and the major extracellular proteases and other hydrolytic enzymes they produce. Pseudoalteromonas, Bacillus, Sulfitobacter and Salegentibacter were dominant taxa in the communities, with all isolates capable of producing serine and/or metallo-proteases. In addition, most bacteria effectively degraded casein and/or gelatin, and only a small portion exhibited elastinolytic ability. Albirhodobacter, Fictibacillus, Citricoccus, Brachybacterium and Arenibacter were first time to be reported for protease production. Interestingly, all Salegentibacter strains exhibited the highest proteolytic activities for all the three protein substrates casein, gelatin and elastin, and they may be candidates for novel proteases. Through the detection of other extracellular enzyme activities, such as alginase, lipase, amylase and cellulase and nitrate reduction, we established cultures that strongly produce extracellular enzymes with biotechnological application potentials. Furthermore, this study broadened understanding and knowledge on the potential ecological functions of protease-producing bacteria in marine sediments.
Acknowledgements
We are grateful to Xiaoke Hu for helping us to revise the manuscript.
-
Figure 2. Phylogenetic tree of the protease-producing bacteria isolated from the Bohai Bay based on 16S rRNA gene sequences. Taxa and GenBank accession numbers in boldface were generated in this study. The tree was constructed by neighbor-joining method using MEGA version 6.0. Only bootstrap values greater than 50% are presented in the nodes. The scale bar represents 2% nucleotide substitution.
Table 1. Characteristics of the sampling stations and the distribution of different genera in these stations
Properties Station BH07 BH12 BH17 BH18 BH20 BH22 CFD39 GPS 38.35°N, 118.75°E 38.55°N, 117.95°E 38.55°N, 118.95°E 38.75°N, 118.95°E 38.75°N, 118.55°E 38.75°N, 118.15°E 39.07°N, 118.53°E Characteristics for sediment samples Depth/m 16.14 9.66 22.65 25.57 23.78 17.27 12.19 Temperature/°C 24.97 27.11 20.36 19.32 23.27 25.47 26.14 pH 8.11 8.08 7.91 7.90 8.01 8.18 8.08 DO 4.02 4.17 2.20 2.28 3.50 5.11 4.86 Sal 30.30 30.02 30.31 30.45 30.51 30.20 30.63 OrgC/% 0.42 0.51 0.55 0.60 0.54 0.57 0.87 OrgN/% 0.06 0.08 0.09 0.08 0.09 0.10 0.11 C/N1) 7.00 6.38 6.11 7.5 6.00 5.70 7.91 Genera distribution Proteobacteria Pseudoalteromonas 3 8 15 6 16 7 8 Shewanella 3 1 Marinobacter 1 3 Sulfitobacter 4 4 Celeribacter 1 1 1 Albirhodobacter 1 1 Firmicutes Bacillus 7 1 1 Halobacillus 1 1 1 Fictibacillus 1 Actinobacteria Micrococcus 1 1 Citricoccus 1 Brachybacterium 1 Bacteroidetes Arenibacter 2 Salegentibacter 2 2 2 Total strain number (109) Diversity index Shannon–Wiener (H′) 1.58 1.22 1.66 1.01 0.34 0.94 0.64 Simpson (D) 0.72 0.61 0.71 0.61 0.20 0.48 0.34 Pielou (J) 0.81 0.76 0.76 0.92 0.50 0.68 0.58 Note: 1) C/N is the abbreviation of OrgC/OrgN. Table 2. Summary of the inhibition test and the extracellular enzyme production analyses of the tested strains isolated from the Bohai Bay
Genera Strain Inhibition ratio1) (I)/% H/C ratio2) Nitrate PMSF O-P E-64 P-A Casein Gelatin Elastin Amylase Cellulase Alginase Tween 80 Pseudoalteromonas 70004 43.64 22.87 0 0 1.77 3.00 1.27 0 0 0 0 – 70006 60.97 0 3.35 0 2.78 2.92 1.40 4.00 2.18 3.26 6.31 + 70032 80.75 46.83 0 0 1.54 3.10 0 0 0 0 1.70 70038 16.89 0 0 0 2.33 4.50 0 0 0 0 1.61 – 70039 43.83 0 0 0 2.50 4.71 0 0 3.68 1.11 3.27 70048 48.41 16.53 0 0 2.78 5.00 0 0 0 0 4.52 + 70051 71.35 45.19 0 2.99 3.25 2.67 1.86 0 0 0 2.59 – 70053 76.43 29.47 0 0 2.25 5.00 0 0 0 1.49 5.40 – 70056 80.05 0 0 0 2.78 4.14 0 0 0 3.60 0 – 70305 46.37 0 0 0 3.42 1.40 2.21 1.59 0 1.20 3.17 – 70306 49.18 34.79 4.11 0 2.87 3.22 1.58 1.76 1.08 1.25 1.51 + 70307 81.10 44.84 0 0 2.94 2.56 1.92 3.23 0 1.33 3.36 – 70308 99.23 48.20 5.80 0 2.75 2.87 1.58 2.07 0 1.16 3.83 + 70320 52.13 5.81 0 0 2.31 1.46 1.55 1.49 1.22 1.22 1.51 – 70325 39.57 6.21 0 0 3.14 1.27 1.70 1.98 0 1.44 2.99 + 70326 81.42 34.55 0 0 3.19 1.14 2.10 2.19 0 1.96 1.70 – 70333 29.63 6.34 0 0 2.62 1.20 1.55 1.64 0 1.20 2.79 – 70339 90.13 31.28 4.48 4.48 2.00 0 1.50 1.61 4.11 1.23 3.46 + 70342 85.92 3.20 0 0 2.18 1.45 1.58 0 0 0 0 – 70344 70.04 53.84 7.04 0 2.38 1.42 1.25 1.34 0 1.28 2.96 – 70354 51.04 20.35 4.25 0 3.43 2.85 1.73 1.26 0 1.32 1.33 – 70356 68.40 10.60 0 7.72 3.18 1.57 2.09 1.75 0 1.31 1.19 – 70359 77.04 42.95 0 3.74 3.42 1.80 1.60 1.83 0 1.86 2.64 + 70361 50.74 6.17 3.60 0 3.00 2.36 1.77 1.35 0 2.12 2.83 – 70365 92.29 27.77 0 0 3.21 3.29 2.50 1.34 0 2.18 0 + 70369 57.37 34.39 0 0 2.44 2.08 1.50 1.76 1.41 2.37 1.18 – 70373 95.19 44.79 0 3.12 3.29 3.17 1.50 2.97 1.12 3.36 2.73 + 70374 76.82 37.19 0 0 3.13 2.27 1.36 2.36 0 6.55 2.78 – 70375 61.08 0 2.23 0 2.93 2.88 2.11 2.89 0 4.63 0 – 70376 15.39 5.92 0 0 3.00 2.75 1.33 0 0 2.16 0 – 70379 42.46 51.17 3.70 0 2.80 2.64 1.80 1.27 0 3.29 2.79 – 70380 91.52 51.24 1.28 4.57 2.82 3.15 1.64 0 1.59 2.10 0 – 70413 99.18 32.76 0 0 2.29 1.27 1.33 1.40 0 2.62 2.71 – Bacillus 70013 63.69 8.67 0 0 1.80 3.67 0 0 0 8.74 0 – 70035 9.42 0 0 0 1.40 6.50 0 0 0 0 0 + 70037 19.80 3.89 0 0 2.14 7.00 0 0 0 2.96 2.04 – 70049 92.58 44.74 0 3.09 4.80 5.42 1.32 0 0 0 0 – 70055 31.98 18.38 7.45 7.63 1.36 3.86 0 0 0 0 0 – Fictibacillus 70014 43.21 1.84 0 0 1.67 5.00 0 0 0 2.52 0 – Micrococcus 70025 68.60 24.30 7.83 0 4.00 5.17 0 0 0 0 0 – 70027 61.26 17.12 9.01 0 3.80 4.14 0 0 0 1.88 0 – Halobacillus 70074 38.71 0 0 0 3.57 3.33 2.29 0 0 2.95 0 – 70075 63.57 15.94 0 0 2.00 4.00 0 0 1.37 0 0 – Salegentibacter 70304 35.37 6.19 7.24 0 4.60 1.29 5.00 0 0 2.63 0 + 70390 84.24 0 0 0 8.33 7.83 5.40 0 0 3.41 0 + 70400 42.39 0 0 0 5.00 4.60 3.57 0 0 2.00 0 – 70401 46.26 8.49 0 0 6.75 6.00 5.00 0 0 2.62 0 + 70416 20.22 1.31 0 0 6.00 3.00 5.50 0 0 2.11 0 + Marinobacter 70434 41.70 37.85 0 0 2.09 0 0 3.54 0 5.27 2.40 + 70435 38.74 35.58 0 0 1.28 3.19 0 3.82 0 7.84 2.40 + Note: 1) Inhibition ratio (I, %) was calculated by using control activity minus the relative activity of a sample with an inhibitor and the activity of a sample without any inhibitor was taken as a control. 2) H/C ratio is the ratio of the hydrolytic zone diameter versus the colony diameter of a colony on the plate. PMSF, phenylmethylsulfonyl fluoride; OP, 1, 10-phenanthroline; P-A, pepstatin A. -
Akagawa-Matsushita M, Matsuo M, Koga Y, et al. 1993. Alteromonas atlantica sp. nov. and Alteromonas carrageenovora sp. nov., bacteria that decompose algal polysaccharides. International Journal of Systematic and Bacteriology, 43(2): 400–400. doi: 10.1099/00207713-43-2-400 Alasalvar C, Taylor K D A, Zubcov E, et al. 2002. Differentiation of cultured and wild sea bass (Dicentrarchus labrax): Total lipid content, fatty acid and trace mineral composition. Food Chemistry, 79(2): 145–150. doi: 10.1016/S0308-8146(02)00122-X Anastasakis K, Ross A B, Jones J M. 2011. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel, 90(2): 598–607. doi: 10.1016/j.fuel.2010.09.023 Bowman J P. 2007. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Marine Drugs, 5(4): 220–241. doi: 10.3390/md504220 Chen Xiulan, Zhang Yuzhong, Gao Peiji, et al. 2003. Two different proteases produced by a deep-sea psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913. Marine Biology, 143(5): 989–993. doi: 10.1007/s00227-003-1128-2 Chi W J, Park J S, Kang D K, et al. 2014. Production and characterization of a novel thermostable extracellular agarase from Pseudoalteromonas hodoensis newly isolated from the West Sea of South Korea. Applied Biochemistry and Biotechnology, 173(7): 1703–1716. doi: 10.1007/s12010-014-0958-3 Dang Hongyue, Zhu Hu, Wang Jing, et al. 2009. Extracellular hydrolytic enzyme screening of culturable heterotrophic bacteria from deep-sea sediments of the Southern Okinawa Trough. World Journal of Microbiology and Biotechnology, 25(1): 71–79. doi: 10.1007/s11274-008-9865-5 Demirbas A, DemiRbas M F. 2011. Importance of algae oil as a source of biodiesel. Energy Conversion and Management, 52(1): 163–170. doi: 10.1016/j.enconman.2010.06.055 Engel A S, Porter M L, Stern L A, et al. 2004. Bacterial diversity and ecosystem function of filamentous microbial mats from aphotic (cave) sulfidic springs dominated by chemolithoautotrophic “Epsilonproteobacteria”. FEMS Microbiology Ecology, 51(1): 31–53. doi: 10.1016/j.femsec.2004.07.004 Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39(4): 783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x Feng Huan, Jiang Hongyou, Gao Wensheng, et al. 2011. Metal contamination in sediments of the western Bohai Bay and adjacent estuaries, China. Journal of Environmental Management, 92(4): 1185–1197. doi: 10.1016/j.jenvman.2010.11.020 Fu Yanzhao, Xu Shiguo, Liu Jianwei. 2016. Temporal-spatial variations and developing trends of Chlorophyll-a in the Bohai Sea, China. Estuarine, Coastal and Shelf Science, 173: 49–56. doi: 10.1016/j.ecss.2016.02.016 Gamal R F, El-Tayeb T S, Raffat E I, et al. 2016. Optimization of chitin yield from shrimp shell waste by Bacillus subtilis and impact of gamma irradiation on production of low molecular weight chitosan. International Journal of Biological Macromolecules, 91: 598–608. doi: 10.1016/j.ijbiomac.2016.06.008 Hansen G H, Sørheim R. 1991. Improved method for phenotypical characterization of marine bacteria. Journal of Microbiological Methods, 13(3): 231–241. doi: 10.1016/0167-7012(91)90049-V He Peiqing, Li Li, Liu Jihua, et al. 2016. Diversity and distribution of catechol 2, 3-dioxygenase genes in surface sediments of the Bohai Sea. FEMS Microbiology Letters, 363(10): fnw086. doi: 10.1093/femsle/fnw086 Hill T C J, Walsh K A, Harris J A, et al. 2003. Using ecological diversity measures with bacterial communities. FEMS Microbiology Ecology, 43(1): 1–11. doi: 10.1111/j.1574-6941.2003.tb01040.x Hu Ningjing, Shi Xuefa, Liu Jihua, et al. 2010. Concentrations and possible sources of PAHs in sediments from Bohai Bay and adjacent shelf. Environmental Earth Sciences, 60(8): 1771–1782. doi: 10.1007/s12665-009-0313-0 Hunter E M, Mills H J, Kostka J E. 2006. Microbial community diversity associated with carbon and nitrogen cycling in permeable shelf sediments. Applied and Environmental Microbiology, 72(9): 5689–5701. doi: 10.1128/AEM.03007-05 Itoh T. 1990. Cellulose synthesizing complexes in some giant marine algae. Journal of Cell Science, 95(2): 309–319 Kitamikado M, Yamaguchi K, Tseng C H, et al. 1990. Method designed to detect alginate-degrading bacteria. Applied and Environmental Microbiology, 56(9): 2939–2940. doi: 10.1128/AEM.56.9.2939-2940.1990 Li Yan, Wu Chaoya, Zhou Mingyang, et al. 2017. Diversity of cultivable protease-producing bacteria in Laizhou Bay sediments, Bohai Sea, China. Frontiers in Microbiology, 8: 405. doi: 10.3389/fmicb.2017.00405 Mondol M M A M, Shin H J, Islam M T. 2013. Diversity of secondary metabolites from marine Bacillus species: Chemistry and biological activity. Marine Drugs, 11(8): 2846–2872. doi: 10.3390/md11082846 Mu Di, Yuan Dekui, Feng Huan, et al. 2017. Nutrient fluxes across sediment-water interface in Bohai Bay Coastal Zone, China. Marine Pollution Bulletin, 114(2): 705–714. doi: 10.1016/j.marpolbul.2016.10.056 Olivera N L, Sequeiros C, Nievas M L. 2007. Diversity and enzyme properties of protease-producing bacteria isolated from sub-Antarctic sediments of Isla de Los Estados, Argentina. Extremophiles, 11(3): 517–526. doi: 10.1007/s00792-007-0064-3 Pujalte M J, Sitjà-Bobadilla A, Macián M C, et al. 2007. Occurrence and virulence of Pseudoalteromonas spp. in cultured gilthead sea bream (Sparus aurata L.) and European sea bass (Dicentrarchus labrax L.). Molecular and phenotypic characterisation of P. undina strain U58. Aquaculture, 271(1–4): 47–53. doi: 10.1016/j.aquaculture.2007.06.015 Putz M, Schleusner P, Rütting T, et al. 2018. Relative abundance of denitrifying and DNRA bacteria and their activity determine nitrogen retention or loss in agricultural soil. Soil Biology and Biochemistry, 123: 97–104. doi: 10.1016/j.soilbio.2018.05.006 R Core Team. 2014. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2013 Saitou N, Nei M. 1987. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4(4): 406–425. doi: 10.1093/oxfordjournals.molbev.a040454 Sanger F, Nicklen S, Coulson A R. 1977. DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 74(12): 5463–5467. doi: 10.1073/pnas.74.12.5463 Sfanos K, Harmody D, Dang P, et al. 2005. A molecular systematic survey of cultured microbial associates of deep-water marine invertebrates. Systematic and Applied Microbiology, 28(3): 242–264. doi: 10.1016/j.syapm.2004.12.002 Smant G, Stokkermans J P W G, Yan Yitang, et al. 1998. Endogenous cellulases in animals: Isolation of β-1, 4-endoglucanase genes from two species of plant-parasitic cyst nematodes. Proceedings of the National Academy of Sciences of the United States of America, 95(9): 4906–4911. doi: 10.1073/pnas.95.9.4906 Sun Jinsheng, Guo Fei, Geng Xuyun, et al. 2011. Seasonal changes and diversity of bacteria in Bohai Bay by RFLP analysis of PCR-amplified 16S rDNA gene fragments. World Journal of Microbiology and Biotechnology, 27(2): 275–284. doi: 10.1007/s11274-010-0456-x Tamura K, Stecher G, Peterson D, et al. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12): 2725–2729. doi: 10.1093/molbev/mst197 Thamdrup B, Dalsgaard T. 2008. Nitrogen cycling in sediments. In: Kirchman D L, ed. Microbial Ecology of the Oceans. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc., 527–568 Tsekos I. 1999. The sites of cellulose synthesis in algae: Diversity and evolution of cellulose-synthesizing enzyme complexes. Journal of Phycology, 35(4): 635–655. doi: 10.1046/j.1529-8817.1999.3540635.x Viola R, Nyvall P, Pedersén M. 2001. The unique features of starch metabolism in red algae. Proceedings of the Royal Society B: Biological Sciences, 268(1474): 1417–1422. doi: 10.1098/rspb.2001.1644 Yadav A N, Sachan S G, Verma P, et al. 2015. Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. Journal of Basic Microbiology, 56(3): 294–307. doi: 10.1002/jobm.201500230 Zhang Xiying, Han Xiaoxu, Chen Xulan, et al. 2015. Diversity of cultivable protease-producing bacteria in sediments of Jiaozhou Bay, China. Frontiers in Microbiology, 6: 1021. doi: 10.3389/fmicb.2015.01021 Zhang Quansheng, Tang Xuexi, Cong Yizhou, et al. 2007. Breeding of an elite Laminaria variety 90–1 through inter-specific gametophyte crossing. Journal of Applied Phycology, 19(4): 303–311. doi: 10.1007/s10811-006-9137-4 Zhao Huilin, Chen Xiulan, Xie Binbin, et al. 2012. Elastolytic mechanism of a novel M23 metalloprotease pseudoalterin from deep-sea Pseudoalteromonas sp. CF6–2: Cleaving not only glycyl bonds in the hydrophobic regions but also peptide bonds in the hydrophilic regions involved in cross-linking. Journal of Biological and Chemistry, 287(47): 39710–39720. doi: 10.1074/jbc.M112.405076 Zhou Mingyang, Chen Xiulan, Zhao Huilin, et al. 2009. Diversity of both the cultivable protease-producing bacteria and their extracellular proteases in the sediments of the South China Sea. Microbial Ecology, 58(3): 582–590. doi: 10.1007/s00248-009-9506-z Zhou Mingyang, Wang Guanglong, Li Dan, et al. 2013. Diversity of both the cultivable protease-producing bacteria and bacterial extracellular proteases in the coastal sediments of King George Island, Antarctica. PLoS One, 8(11): e79668. doi: 10.1371/journal.pone.0079668 -

 点击查看大图
点击查看大图
计量
- 文章访问数: 826
- HTML全文浏览量: 260
- 被引次数: 0




 DownLoad:
DownLoad:
 下载:
下载:


