| Citation: | Chunyan Zhao, Liang Chi, Yongshuang Xiao, Bing Li, Yunliang Lu, Yanting Cui, Wenqi Wang, Jun Li. Morphological and histological changes in the brains of turbot (Scophthalmus maximus) with gonadal development[J]. Acta Oceanologica Sinica, 2022, 41(12): 115-122. doi: 10.1007/s13131-022-2041-1 |
Fish brains comprised of several main structures, they are olfactory bulbs, telencephalon, mesencephalon, diencephalon (including the optic tectum and hypothalamus), cerebellum, dorsal medulla, and pituitary gland (Broglio et al., 2003; Kotrschal et al., 1998; Ullmann et al., 2010). In most fish, the telencephalon consists of paired cerebral hemispheres with olfactory bulbs attached to the rostral hemispheres (Striedter and Northcutt, 2006). The mesencephalon and diencephalon possess tegmentum and overlie the spinal cord. The cerebellum arises from the rostral roof, and the myelencephalon is positioned behind the cerebellum. The hypothalamus and pituitary gland are located on the ventral side of the brain. The inferior lobes of the hypothalamus are paired, and the hypothalamic tegmentum serves to convert sensory inputs into hormonal and behavioral responses. The saccus vasculosus contains cerebrospinal fluid that contacts neurons and the distinctive ependyma (Kotrschal et al., 1983). The pituitary gland serves as the central humoral command unit for physiology and behavior and is self-controlled by the hypothalamus.
Reproduction is a fundamental part of life and is necessary for species propagation. The hypothalamus-pituitary-gonadal (HPG) axis plays a critical role in controlling reproduction through functional neuropeptides and hormonal systems in vertebrates (Acevedo-Rodriguez et al., 2018; Bauchot et al., 1989; Dwyer and Quinton, 2019; Loveland et al., 2021). Briefly, the hypothalamic release of gonadotropin-releasing hormone (GnRH) stimulates the secretion of gonadotropic hormones (GtHs), luteinizing hormone (LH), and follicle stimulating hormone (FSH) from the anterior pituitary gonadotropes. When LH and FSH reach the gonads, they stimulate gametogenesis and promote gonadal release of steroids such as testosterone, estradiol, and progesterone. These gonadal steroids can act as a feedback system to modulate upstream HPG components (Bizzarri and Cappa, 2020; MacManes et al., 2017; Maharajan et al., 2020; Meethal and Atwood, 2005). Thus, the HPG axis depends on the brain and pituitary gland to guide proper gonadal development and facilitate reproduction.
Turbot (Scophthalmus maximus) were originally farmed in Europe, but have become one of the most economically important marine species farmed in northern China (Alvariño et al., 2001; Lin et al., 2012; Zhao et al., 2018a). Because artificial reproduction is vital to turbot farming, it is very important to obtain mature eggs and sperm concurrently during the breeding season. There have been some studies on the reproduction of turbot (Imsland et al., 2003; Stoss and Røer, 2020). However, most of these studies focused on testes and ovary development and maturation, including sex differentiation (Zhao et al., 2017) and germ cell differentiation (Lin et al., 2013; Liu et al., 2021; Xue et al., 2018; Zhou et al., 2019). Research investigating the role of central nervous system (CNS) organs, particularly regarding the brain and pituitary gland, is rare. The only report on GnRH in brain areas and gonads of male and female turbots showed direct regulation of gonadal development by the GnRH system (Zhao et al., 2018b). However, the morphology and histology of CNS organs via the HPG axis, in coordinating with gonadal development, have not been studied. Therefore, a comprehensive understanding of organ development in the HPG axis is urgently needed.
The aim of this study is to investigate sex differences in the brains of turbot during gonadal development. Firstly, the characteristics of the turbot brain were described using anatomy and histology. Next, changes in morphology of the pituitary gland were measured as volume at different stages of gonadal development. These results will help elucidate significant links between turbot brain structure and gonadal development.
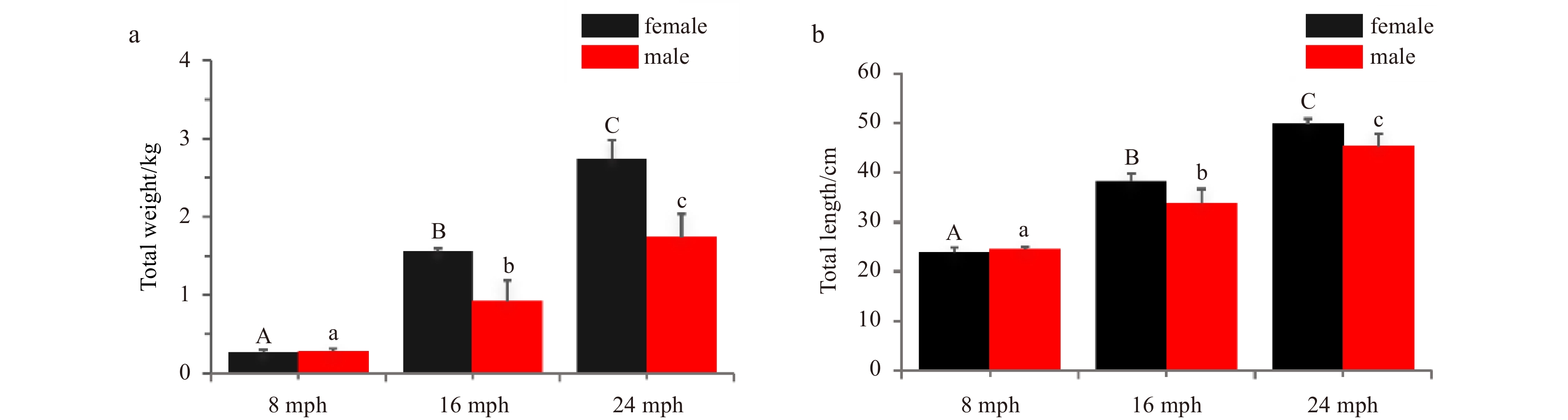
Turbots were obtained from Laiyang aquafarm (Shandong, China) according to their developmental stages. Samples included male and female fish at 8 (immature), 16 (maturing), and 24 (mature) months post-hatching (mph). Fifteen male and fifteen female turbots were sampled for each stage, and their total weight and length were measured (Fig. 1). The whole brain and gonads were collected after the fish were anesthetized in 200 g/L MS-222 (Sigma, Saint Louis, MO, USA). The brains were fixed in 4% paraformaldehyde for 24 h for morphological assessment. After photographs were obtained using the SteREO Discovery V20 microscope (Zeiss, Oberkochen, Germany), the brain samples were used for histological analysis. The gonads were submerged in 4% paraformaldehyde for 24 h for fixing and stored in 70% ethyl alcohol until histological processing.
In this study, the volumes of the whole brain and distinct brain regions were evaluated. The volumes (V) of all brain structures were calculated according to an idealized ellipsoid model (Pollen et al., 2007) following the formula V=(L×W×H) π/6. The width (W) and length (L) were measured using dorsal and ventral images, and the height (H) was measured using lateral images according to the method shown in Fig. 2. For paired structures, the estimated volume was doubled. In addition, the total brain volume was estimated using the ellipsoid method. The length of the total brain was defined as from the telencephalon to the medulla, the height was defined as from the midbrain to the hypothalamus, and the width was defined as across the two-optic tecta.

Fixed brain and gonad samples were dehydrated using an incremental series of ethanol washes, clarified in xylene, embedded in paraffin, and sliced into serial transverse sections at 5 μm using a rotary microtome (Leica, Wetzlar, Germany). The sections were stained with haematoxylin and eosin (HE) and examined using an Axio Scope A1 microscope (Leica, Wetzlar, Germany).
All results are expressed as mean±SD. Statistics were performed using SPSS 15.0 software. Data were compared using two-way ANOVA, and significant differences were detected using Duncan’s multiple range test.
In gross view, the brains of turbots were attached to the anteroventral aspect of the body and behind the eyes (Figs 3a, b). The whole brains contained seven parts: telencephalon, diencephalon, cerebellum, hypothalamus, pituitary gland, myelencephalon, and olfactory bulbs (Figs 3c–e). The telencephalon was pronounced, with prominent lobules. The diencephalon was located to the posterior of the telencephalon and was large. The cerebellum was dorsally expanded and caudally directed, and was relatively large. The olfactory bulbs were positioned rostrally to the telencephalon.
The brains of turbots were characterized as having a typical advanced teleost brain architecture, with unique features (Figs 4 and 5).


The telencephalon was clear and consisted of five distinct lobes including the dorsodorsal nucleus (Dd), dorsomedial nucleus (Dm), dorsolateral nucleus (Dl), and dorsocentral nucleus (Dc), with the dorsolateral nucleus (Dp) positioned in the ventral division. The lobation was clear and distinct above the anterior commissure (Ac). The ventral posterior nucleus (Vp) was located in the caudal-most telencephalon (Fig. 4a). The caudal portions of the olfactory bulbs (OB) were positioned under the telencephalon.
The diencephalon was contiguous with the caudal portion of the telencephalon, with a distinct habenulae (Ha) and dorsal medial nucleus of the thalamus (DM). Magnocellular preoptic nuclei (PP) were abundant in the dorsal preoptic area. Narrow subependymal expansions were present at the posterior commissure. The thalamic nuclei of the dorsoposterior, centroposterior nuclei, the hypothalamic nuclei of the dorsal nuclei, and ventral nuclei all appeared clearly in their normal positions. The nucleus glomerulosus (G) was large and circular. The inferior lobes were medium sized. The lateral recess (LR) was positioned within the lobe. The cells of the nucleus diffusus (ND) were sparse, but well-organized. The mammillary bodies (CM) met along the third ventricle, and the saccus vasculosus was evident (Figs 4b–d).
The tectum of mesencephalon (Tec) was circular and the superficial white matter was proportional and apparent. The pretectal nucleus (NPTec) was large. The torus longitudinales (Tl) and rostral torus semicircularis (Ts) were present, and were flattened along the dorsal surface. The medial longitudinal fasciculus (MLF) was always apparent.
The valvula cerebellum (Vcb) (Figs 4c, d) appeared in the mesocoel and included a molecular layer and granule cell regions. The valvula initially formed as single lobes, becoming broader in the caudal direction with two large and stacked lobes. The rostral corpus cerebellum (CCb) was located in the dorsal portion of the brain (Figs 5a–d) and was tall and broad. The eminentia granulares (EG) and motor nucleus of the trigeminal nerve (Vm) appeared clearly.
Further in the caudal direction, the crista cerebellaris (CC) was prominent (Figs 5c, d), but decreased in size from the rostral to caudal ends.
The distinct brain morphology of both sexes at 8, 16 and 24 mph are presented in Fig. 6. The pituitary gland increased significantly in volume as body size increased. Though the size of the pituitary glands at 8 mph did not differ between male and female turbots, the pituitary glands of males grew faster than those of females. This is evidenced by their larger volumes at 16 mph and 24 mph (Figs 6c, f).

The actual volumes of the seven brain structures were investigated at 8 mph, 16 mph, and 24 mph (Figs 7a–c) in both sexes. At 8 mph (Fig. 7a), the mesencephalons of females were larger than those of males, while the hypothalamus and pituitary glands did not differ between the sexes. At 16 mph (Fig. 7b), the male mesencephalon, telencephalon, and pituitary glands were significantly larger than those of the females (p<0.05). At 24 mph (Fig. 7c), only the pituitary glands differed significantly (p<0.05) between females and males, and the males’ pituitary glands were larger (p<0.05) than those of the females. The size of the telencephalon, cerebellum, myelencephalon, and olfactory bulbs did not differ significantly between females and males at any growth stage. The total brain volumes were also analyzed (Fig. 7d). Male brain volume at 24 mph ((295.83±23.31) mm3) was significantly greater than female brain volume (p<0.05), but there were no significant differences at 8 mph or 16 mph.

The relative volumes of the brain structures were also analyzed. Studies that have compared brain volumes have demonstrated that brain size exhibits an allometric relationship with body size (Gonzalez-Voyer et al., 2009a, b; White and Brown, 2015a, b). To control for these allometric effects, the relative brain structure volume was calculated as relative pituitary gland volume=pituitary volume/total brain volume. The results are shown in Fig. 8. In females, the relative volumes did not differ significantly with different development stages for any of the analyzed brain structures. In males, significant differences in relative volumes were found in the telencephalon, mesencephalon, cerebellum, hypothalamus, and pituitary gland with different development stages. It is notable that a different pattern of relative volumes of the pituitary gland was observed between males and females with different development stages. And the relative volumes of the pituitary gland increased significantly in males between 8 mph and 24 mph, but it did not increase in females.

Gonadal sections confirmed that 8 mph, 16 mph, and 24 mph were represented immature, maturing, and mature stages of gonadal development, respectively. In immature ovaries, the oocytes were in the chromatin nucleolar and perinucleolus stages (II) (Fig. 9a). The Genadosomatic Index (GSI) of immature females was 0.11±0.02 (Fig. 9d). Immature testes contained both type-A and type-B spermatogonia (Fig. 9e). The GSI of this stage was 0.03±0.008 (Fig. 9h). In maturing ovaries, most oocytes were in the late perinucleolus stage (II) and several oocytes had even entered the vitellogenic stage (III), indicating that vitellogenesis had begun (Fig. 9b). The GSI of this stage was 0.65±0.07 (Fig. 9d). Maturing testes contained primary spermatocytes, secondary spermatocytes, and a small amount of spermatogonia (Fig. 9f). The GSI of this stage was 0.050±0.004 (Fig. 9h). Ovaries at 24 mph were mature, and the oocytes had undergone the late vitellogenic and post-vitellogenic stages (IV) and reached maturation (stage V; Fig. 9c). The GSI of mature females was 5.25±1.93 (Fig. 9d). Testes at the mature stage contained spermatids and spermatozoa (Fig. 9g). The GSI of this stage was 0.48±0.09 (Fig. 9h).

It is well known that different fish brain regions control specific cognitive functions for higher processing and share clear homologies with those of other vertebrates (Broglio et al., 2003; Finger, 1988; White and Brown, 2015a). Several studies have addressed the variability among brains of different species. Each organ serves a different purpose and may develop differently depending on the needs of the fish (Ishikawa et al., 1999; Ito et al., 2007). The telencephalon receives primary olfactory input and integrates it with a variety of other complex sensory inputs (Eastman and Lannoo, 2008; Northcutt, 2006). The tectum is known as a visual center (Demski, 2003). The corpus cerebellum is typically large in teleost fishes and is an important integrative center receiving visual, mechanosensory lateral line, and somatosensory inputs (Ito et al., 2007; Kotrschal et al., 1998). In this study, the turbot had a well-developed and distinct telencephalon, but a less developed cerebellum. This is likely due to their deep-water environment and sedentary lifestyle, which necessitates seeking and locating prey using combined olfactory and visual cues (Briñón et al., 1993; Kotrschal et al., 1998).
Many studies have demonstrated correlations between brain morphology and ecological environments in fish (Burns and Rodd, 2008; White and Brown, 2015a). For example, Pleuronectiform fish are unusual animals in being grossly asymmetric as adults but symmetrical when young. The Pleuronectiforms develop prominent asymmetries only later in development, when they lie either on their left or right body side. Although this change involves a complex modification of the head and neural morphology (Bauchot et al., 1989; Finger, 1988), the associated asymmetries in the brain are somewhat less pronounced than expected. In winter flounder (Pseudopleuronectes americanus), a species of flatfish that is nearly always dextral, i.e., with the eyes on the right side of the head and with its left side facing down, the right telencephalon is approximately 8% larger than the left (Rao and Finger, 1984). The brains of Paraplagusia japonica and Zebrias also exhibit asymmetrical morphologies (Ito et al., 2007). In turbot, the telencephalic hemispheres and the optic tectum developed asymmetries during metamorphosis, but the optic tectum recovered its bilateral symmetry after metamorphosis (Briñón et al., 1993). In this paper, the post metamorphosis turbot brains did not show asymmetries in either the morphological or histological assessments. This may have been because the turbots in this study were adult fish and had already undergone metamorphosis. Thus, more detailed research should investigate the volumes of left and right brain structures to gain a more complete data set describing brain size and structure in turbot.
Previous studies have demonstrated that there are several variations in brain size and structure that stem from adaptive ecology. These variations arise because different vertebrate taxa benefit differently from specific cognitive enhancements. Variables such as habitat complexity, social behavior, diet, parental care strategies, life-history traits, mating strategy, and sexual selection have all been found to correlate with different aspects of brain size and structure (Gonda et al., 2009; Gonzalez-Voyer et al., 2009b; Kolm et al., 2009; Pitnick et al., 2006; Pollen et al., 2007; Safi and Dechmann, 2005; White and Brown, 2015b). For example, comparisons of the brains of precocious and non-precocious fish linked brain size and structure to mating strategy in wild brown trout (Salmo trutta). In addition, the brains of precocious female fish were larger than those of precocious male fish (Gonzalez-Voyer et al., 2009b). However, unlike the brown trout, the female turbots examined in this study had smaller brains than the males, despite the females being larger in total weight and length. This suggests that the size and structure of the turbot brain was driven, to some degree, by sexual selection.
In this study, we first investigated brain changes with gonadal development, and found that pituitary volume increased significantly. In vertebrates, pituitary GtHs play a critical role in the control and regulation of gonadal development, gametogenesis, and gonadal steroidogenesis, and there is a strong correlation between the brain and reproductive behavior, particularly gonadal development (Borella et al., 2019; Loveland et al., 2021). Typical GtH, FSH, and LH cells are distributed in the proximal pars distalis (PPD) and the pars intermedia (PI) areas of adenohypophysis (AH) during early ovarian differentiation and development, indicating they are involved in gonadal development and differentiation (Xu et al., 2020). The distinct pituitary cell types and physiological roles of GtH hormones in turbot may be similar to other teleosts. Furthermore, a hypothesis generated from sexual selection theory suggested that changes in brain size may be accompanied by compensatory changes in sexual organs, showing an evolutionary relationship between investment in testes and brains (Pitnick et al., 2006). Artificially extracted semen from cultured male turbot brood stock is usually of poor quality, with high rates of sperm deformities (Liu et al., 2021). Therefore, the male turbot brood stock, as indicated by their extremely large pituitary glands, may require the synthesis and release of GtHs to improve sperm quality. The pituitary glands consist of two major sections, the neurohypophysis (NH) and the AH, which were investigated using histochemical (HE, Mallory trichrome, and Periodic Acid-Schiff stain) and immunocytochemical approaches (Borella et al., 2009; Miwa and Inui, 1987). The AH was comprised of the pars distalis (PD). However, changes in number of cells and hormone levels were not measured during the gonadal development of males and females in this study. Further studies using multiple techniques should be conducted in order to better understand the pattern and distribution of GtH cells in the pituitary gland, especially in regions producing gonadotropins.
In conclusion, a detailed description of the turbot brain based on morphology and histology was presented in this paper. It was confirmed that the turbot brain architecture is characteristic of advanced teleosts. The total brain volume in mature males was significantly larger than in females. The pituitary gland size grew significantly larger from the immature to the mature stage in males, while there were no differences among developmental stages in females. These data together illustrated a distinct sex difference in the turbot brain during gonadal development. Further research investigating brain function in the HPG axis is needed in order to improve our understanding of gonadal development in turbot brood stock.
|
Acevedo-Rodriguez A, Kauffman A S, Cherrington B D, et al. 2018. Emerging insights into hypothalamic-pituitary-gonadal axis regulation and interaction with stress signalling. Journal of Neuroendocrinology, 30(10): e12590
|
|
Alvariño J M R, Rebollar P G, Olmedo M, et al. 2001. Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquaculture International, 9(6): 477–487. doi: 10.1023/A:1020590111031
|
|
Bauchot R, Ridet J M, Bauchot M L. 1989. The brain organization of butterflyfishes. Environmental Biology of Fishes, 25(1): 205
|
|
Bizzarri C, Cappa M. 2020. Ontogeny of hypothalamus-pituitary gonadal axis and minipuberty: an ongoing debate?. Frontiers in Endocrinology, 11: 187.
|
|
Borella M I, Chehade C, Costa F G, et al. 2019. The brain-pituitary-gonad axis and the gametogenesis. In: Baldisserotto B, Urbinati E C, Cyrino J E P, eds. Biology and Physiology of Freshwater Neotropical Fish. Amsterdam: Academic Press, 315–341
|
|
Borella M I, Venturieri R, Mancera J M. 2009. Immunocytochemical identification of adenohypophyseal cells in the pirarucu (Arapaima gigas), an Amazonian basal teleost. Fish Physiology and Biochemistry, 35(1): 3–16. doi: 10.1007/s10695-008-9254-x
|
|
Briñón J G, Médina M, Arévalo R, et al. 1993. Volumetric analysis of the telencephalon and tectum during metamorphosis in a flatfish, the turbot Scophthalmus maximus. Brain, Behavior and Evolution, 41(1): 1–5
|
|
Broglio C, Rodríguez F, Salas C. 2003. Spatial cognition and its neural basis in teleost fishes. Fish and Fisheries, 4(3): 247–255. doi: 10.1046/j.1467-2979.2003.00128.x
|
|
Burns J G, Rodd F H. 2008. Hastiness, brain size and predation regime affect the performance of wild guppies in a spatial memory task. Animal Behaviour, 76(3): 911–922. doi: 10.1016/j.anbehav.2008.02.017
|
|
Demski L S. 2003. In a fish’s mind’s eye: the visual pallium of teleosts. In: Collin S P, Marshall N J, eds. Sensory Processing in Aquatic Environments. New York: Springer, 404–419
|
|
Dwyer A A, Quinton R. 2019. Anatomy and physiology of the hypothalamic-pituitary-gonadal (HPG) axis. In: Llahana S, Follin C, Yedinak C, et al., eds. Advanced Practice in Endocrinology Nursing. Cham: Springer, 839–852
|
|
Eastman J T, Lannoo M J. 2008. Brain and sense organ anatomy and histology of the Falkland Islands mullet, Eleginops maclovinus (Eleginopidae), the sister group of the Antarctic notothenioid fishes (Perciformes: Notothenioidei). Journal of Morphology, 269(1): 84–103. doi: 10.1002/jmor.10571
|
|
Finger T E. 1988. Organization of chemosensory systems within the brains of bony fishes. In: Atema J, Fay R R, Popper A N, et al., eds. Sensory Biology of Aquatic Animals. New York: Springer, 339–363
|
|
Gonda A, Herczeg G, Merilä J. 2009. Adaptive brain size divergence in nine-spined sticklebacks (Pungitius pungitius)?. Journal of Evolutionary Biology, 22(8): 1721–1726
|
|
Gonzalez-Voyer A, Winberg S, Kolm N. 2009a. Brain structure evolution in a basal vertebrate clade: evidence from phylogenetic comparative analysis of cichlid fishes. BMC Evolutionary Biology, 9(1): 238. doi: 10.1186/1471-2148-9-238
|
|
Gonzalez-Voyer A, Winberg S, Kolm N. 2009b. Social fishes and single mothers: brain evolution in African cichlids. Proceedings of the Royal Society B: Biological Sciences, 276(1654): 161–167. doi: 10.1098/rspb.2008.0979
|
|
Imsland A K, Dragsnes M, Stefansson S O. 2003. Exposure to continuous light inhibits maturation in turbot (Scophthalmus maximus). Aquaculture, 219(1–4): 911–919
|
|
Ishikawa Y, Yoshimoto M, Yamamoto N, et al. 1999. Different brain morphologies from different genotypes in a single teleost species, the Medaka (Oryzias latipes). Brain, Behavior and Evolution, 53(1): 2–9
|
|
Ito H, Ishikawa Y, Yoshimoto M, et al. 2007. Diversity of brain morphology in teleosts: brain and ecological niche. Brain, Behavior and Evolution, 69(2): 76–86
|
|
Kolm N, Gonzalez-Voyer A, Brelin D, et al. 2009. Evidence for small scale variation in the vertebrate brain: mating strategy and sex affect brain size and structure in wild brown trout (Salmo trutta). Journal of Evolutionary Biology, 22(12): 2524–2531. doi: 10.1111/j.1420-9101.2009.01875.x
|
|
Kotrschal K, Krautgartner W D, Adam H. 1983. Crown cells in the diencephalon of Acipenser ruthenus (Acipenseridae, Chondrostei). Journal für Hirnforschung, 24(6): 655–657
|
|
Kotrschal K, van Staaden M J, Huber R. 1998. Fish brains: evolution and anvironmental relationships. Reviews in Fish Biology and Fisheries, 8(4): 373–408. doi: 10.1023/A:1008839605380
|
|
Lin Fan, Xu Shihong, Ma Daoyuan, et al. 2012. Germ line specific expression of a vasa homologue gene in turbot (Scophthalmus maximus): evidence for vasa localization at cleavage furrows in euteleostei. Molecular Reproduction and Development, 79(11): 803–813. doi: 10.1002/mrd.22120
|
|
Lin Fan, Zhao Chunyan, Xu Shihong, et al. 2013. Germline-specific and sexually dimorphic expression of a dead end gene homologue in turbot (Scophthalmus maximus). Theriogenology, 80(6): 665–672. doi: 10.1016/j.theriogenology.2013.06.016
|
|
Liu Yifan, Liu Qinghua, Xu Shihong, et al. 2021. A deep insight of spermatogenesis and hormone levels of aqua-cultured turbot (Scophthalmus maximus). Frontiers in Marine Science, 7: 592880. doi: 10.3389/fmars.2020.592880
|
|
Loveland J L, Giraldo-Deck L M, Lank D B, et al. 2021. Functional differences in the hypothalamic-pituitary-gonadal axis are associated with alternative reproductive tactics based on an inversion polymorphism. Hormones and Behavior, 127: 104877. doi: 10.1016/j.yhbeh.2020.104877
|
|
MacManes M D, Austin S H, Lang A S, et al. 2017. Widespread patterns of sexually dimorphic gene expression in an avian hypothalamic–pituitary–gonadal (HPG) axis. Scientific Reports, 7: 45125. doi: 10.1038/srep45125
|
|
Maharajan K, Muthulakshmi S, Karthik C, et al. 2020. Pyriproxyfen induced impairment of reproductive endocrine homeostasis and gonadal histopathology in zebrafish (Danio rerio) by altered expression of hypothalamus-pituitary-gonadal (HPG) axis genes. Science of the Total Environment, 735: 139496. doi: 10.1016/j.scitotenv.2020.139496
|
|
Meethal S V, Atwood C S. 2005. The role of hypothalamic-pituitary-gonadal hormones in the normal structure and functioning of the brain. Cellular and Molecular Life Sciences, 62(3): 257–270. doi: 10.1007/s00018-004-4381-3
|
|
Miwa S, Inui Y. 1987. Histological changes in the pituitary-thyroid axis during spontaneous and artificially-induced metamorphosis of larvae of the flounder Paralichthys olivaceus. Cell and Tissue Research, 249(1): 117–123. doi: 10.1007/BF00215425
|
|
Northcutt R G. 2006. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. Journal of Comparative Neurology, 494(6): 903–943. doi: 10.1002/cne.20853
|
|
Pitnick S, Jones K E, Wilkinson G S. 2006. Mating system and brain size in bats. Proceedings of the Royal Society B: Biological Sciences, 273(1587): 719–724. doi: 10.1098/rspb.2005.3367
|
|
Pollen A A, Dobberfuhl A P, Scace J, et al. 2007. Environmental complexity and social organization sculpt the brain in Lake Tanganyikan cichlid fish. Brain, Behavior and Evolution, 70(1): 21–39
|
|
Rao P D P, Finger T E. 1984. Asymmetry of the olfactory system in the brain of the winter flounder, Pseudopleuronectes americanus. Journal of Comparative Neurology, 225(4): 492–510. doi: 10.1002/cne.902250403
|
|
Safi K, Dechmann D K N. 2005. Adaptation of brain regions to habitat complexity: a comparative analysis in bats (Chiroptera). Proceedings of the Royal Society B: Biological Sciences, 272(1559): 179–186. doi: 10.1098/rspb.2004.2924
|
|
Stoss J H, Røer J E. 2020. Controlled reproduction in turbot (Scopthalmus maximus). In: Reinertsen H, Dahle L A, Jørgensen L, et al., eds. Fish Farming Technology. London: CRC Press
|
|
Striedter G F, Northcutt R G. 2006. Head size constrains forebrain development and evolution in ray-finned fishes. Evolution and Development, 8: 215-222
|
|
Ullmann J F P, Cowin G, Collin S P. 2010. Quantitative assessment of brain volumes in fish: comparison of methodologies. Brain, Behavior and Evolution, 76(3–4): 261–270
|
|
White G E, Brown C. 2015a. Microhabitat use affects brain size and structure in intertidal gobies. Brain, Behavior and Evolution, 85(2): 107–116
|
|
White G E, Brown C. 2015b. Variation in brain morphology of intertidal gobies: a comparison of methodologies used to quantitatively assess brain volumes in fish. Brain, Behavior and Evolution, 85(4): 245–256
|
|
Xu Wengang, Manabe S, Mushirobira Y, et al. 2020. Changes in expression of reproduction-related hormones in the brain and pituitary during early ovarian differentiation and development in the red spotted grouper Epinephelus akaara, with emphasis on FSHβ and LHβ. Aquaculture, 514: 734497. doi: 10.1016/j.aquaculture.2019.734497
|
|
Xue Rui, Wang Xueying, Xu Shihong, et al. 2018. Expression profile and localization of vitellogenin mRNA and protein during ovarian development in turbot (Scophthalmus maximus). Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 226: 53–63. doi: 10.1016/j.cbpb.2018.08.002
|
|
Zhao Chunyan, Liu Qinghua, Xu Shihong, et al. 2018a. Identification of type A spermatogonia in turbot (Scophthalmus maximus) using a new cell-surface marker of Lymphocyte antigen 75 (ly75/CD205). Theriogenology, 113: 137–145. doi: 10.1016/j.theriogenology.2017.12.016
|
|
Zhao Chunyan, Xu Shihong, Feng Chengcheng, et al. 2018b. Characterization and differential expression of three GnRH forms during reproductive development in cultured turbot Schophthalmus maximus. Journal of Oceanology and Limnology, 36(4): 1360–1373. doi: 10.1007/s00343-018-7068-y
|
|
Zhao Chunyan, Xu Shihong, Liu Yifan, et al. 2017. Gonadogenesis analysis and sex differentiation in cultured turbot (Scophthalmus maximus). Fish Physiology and Biochemistry, 43(1): 265–278. doi: 10.1007/s10695-016-0284-5
|
|
Zhou Li, Wang Xueying, Liu Qinghua, et al. 2019. Visualization of turbot (Scophthalmus maximus) primordial germ cells in vivo using fluorescent protein mediated by the 3′ untranslated region of nanos3 or vasa gene. Marine Biotechnology, 21(5): 671–682. doi: 10.1007/s10126-019-09911-z
|
