Comparative mitochondrial genome analysis of Varunidae and its phylogenetic implications
-
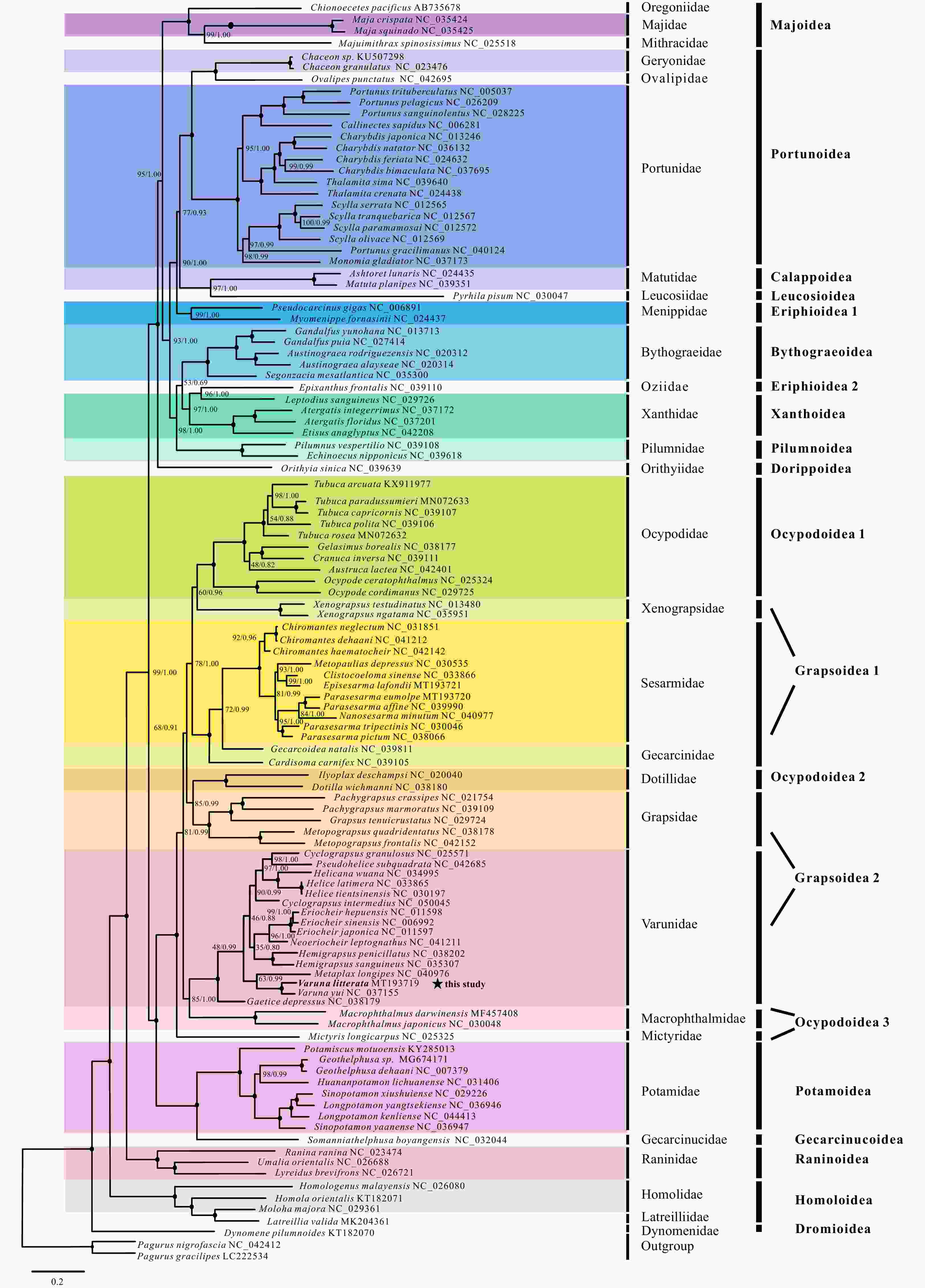
Abstract: Complete mitochondrial genomes (mitogenomes) can indicate phylogenetic relationships, as well as useful information for gene rearrangement mechanisms and molecular evolution. Currently, the phylogenetic location of the genus Varuna (Brachyura: Varunidae) has not been well resolved mainly because of limited representatives (only two extant species). Here, we determined a new mitogenome of this genus (Varuna litterata) and added the published mitogenomes to reconstruct the phylogeny of Varunidae. The 16 368-bp mitogenome contains the entire set of 37 genes and a putative control region. The characteristics of this newly sequenced mitogenome were described and compared with the other 15 Varunidae mitogenomes. All 16 analyzed mitogenomes have identical gene order and similar molecular features. The sliding window and genetic distance analyses demonstrate highly variable nucleotide diversity, with comparatively low variability of COI and COII, and high variability of ND6. The nonsynonymous/synonymous substitution rates (dN/dS ratio) analysis shows that all 13 PCGs are under purifying selection and ATP8 gene evolves under the least selective pressure. Twelve tRNA genes, two rRNAs, one PCG, and the putative control region are found to be rearranged with respect to the pancrustacean ground pattern gene order. Tandem duplication/random loss model is adopted to explain the large-scale gene rearrangement events occurring in Varunidae mitogenomes. Phylogenetic analyses show that all Varunidae species are placed into one group, and form a sister clade with Macrophthalmidae. Nevertheless, the phylogenetic relationships within Varunidae are not completely consistent based on the two different datasets used in this study. These findings will contribute to a better understanding of gene rearrangement and molecular evolution in Varunidae mitogenomes, as well as provide insights into the phylogenetic studies of Brachyura.
-
Key words:
- varunid crab /
- Varuna litterata /
- mitogenome /
- gene rearrangement /
- tandem duplication/random loss /
- phylogeny
-
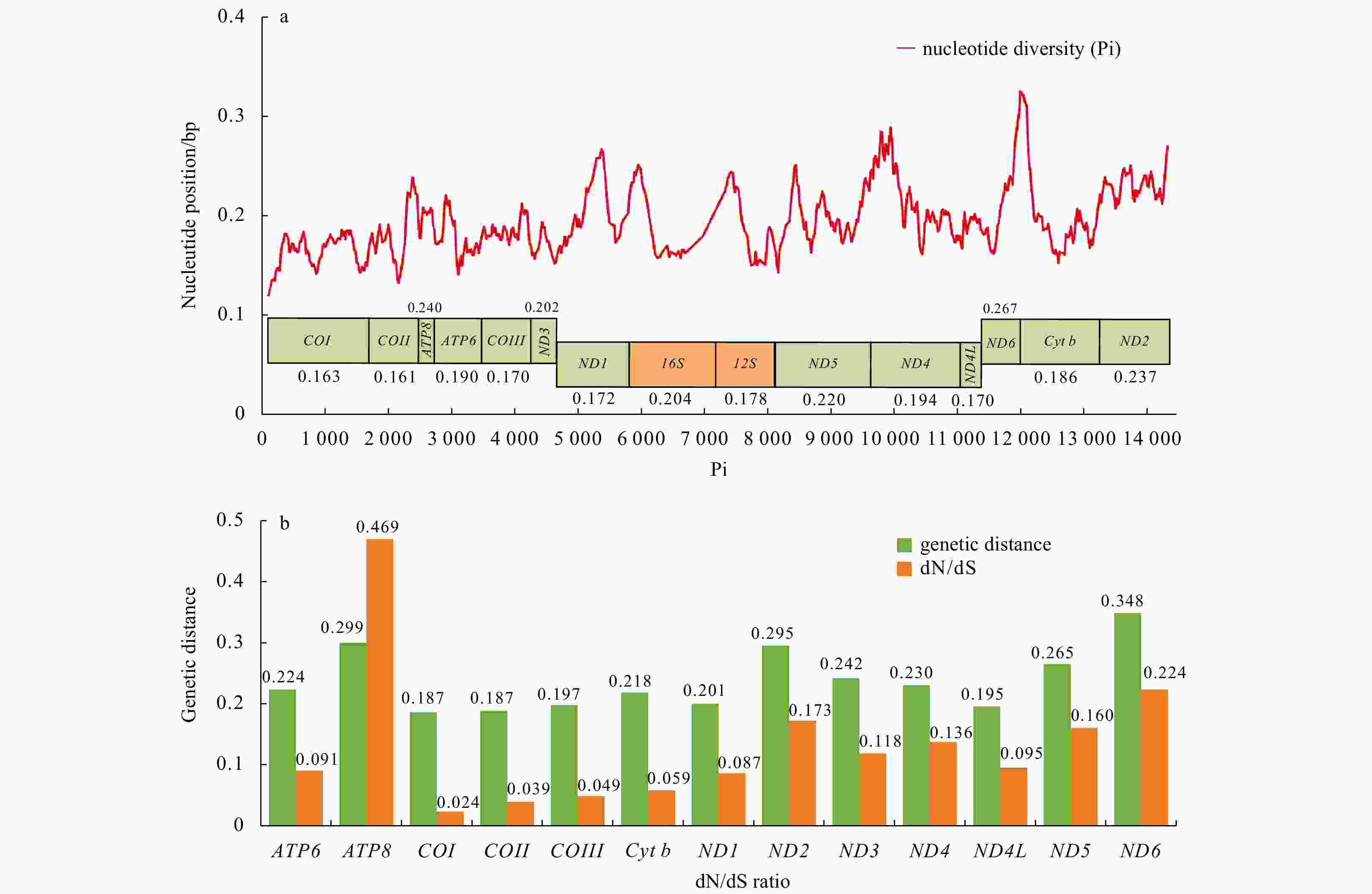
Figure 3. Sliding window analyses of 13 PCGs (green box) and 2 rRNAs (orange box) among 16 Varunidae mitogenomes (a). The red line shows the value of nucleotide diversity (Pi) in a sliding window analysis (a sliding window of 200 bp with a step size of 20 bp). Gene names and the Pi value of each gene are indicated above or below the graph. Genetic distance (on average) and nonsynonymous/synonymous substitution rates (dN/dS) of 13 PCGs among 16 Varunidae species (b).
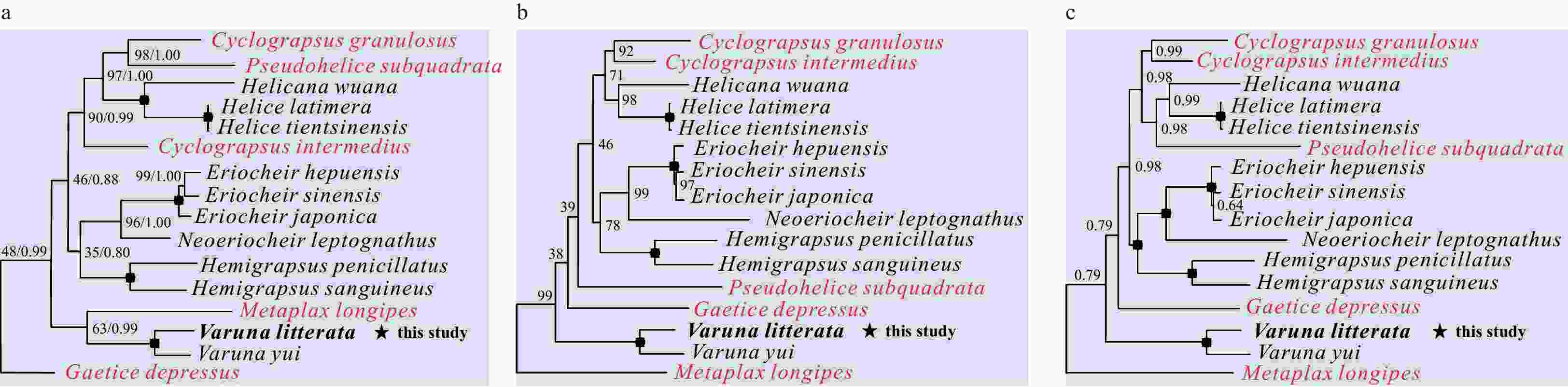
Figure 4. Phylogenetic trees of Varunidae species inferred from 13 PCGs based on different methods. a. Nucleotide sequences based on maximum likelihood (ML) and Bayesian inference (BI) analysis; b. amino acid sequences based on ML analysis; c. amino acid sequences based on BI analysis. Node marked with a solid circle indicates 100 maximum likelihood bootstrap value and 100% supporting value.
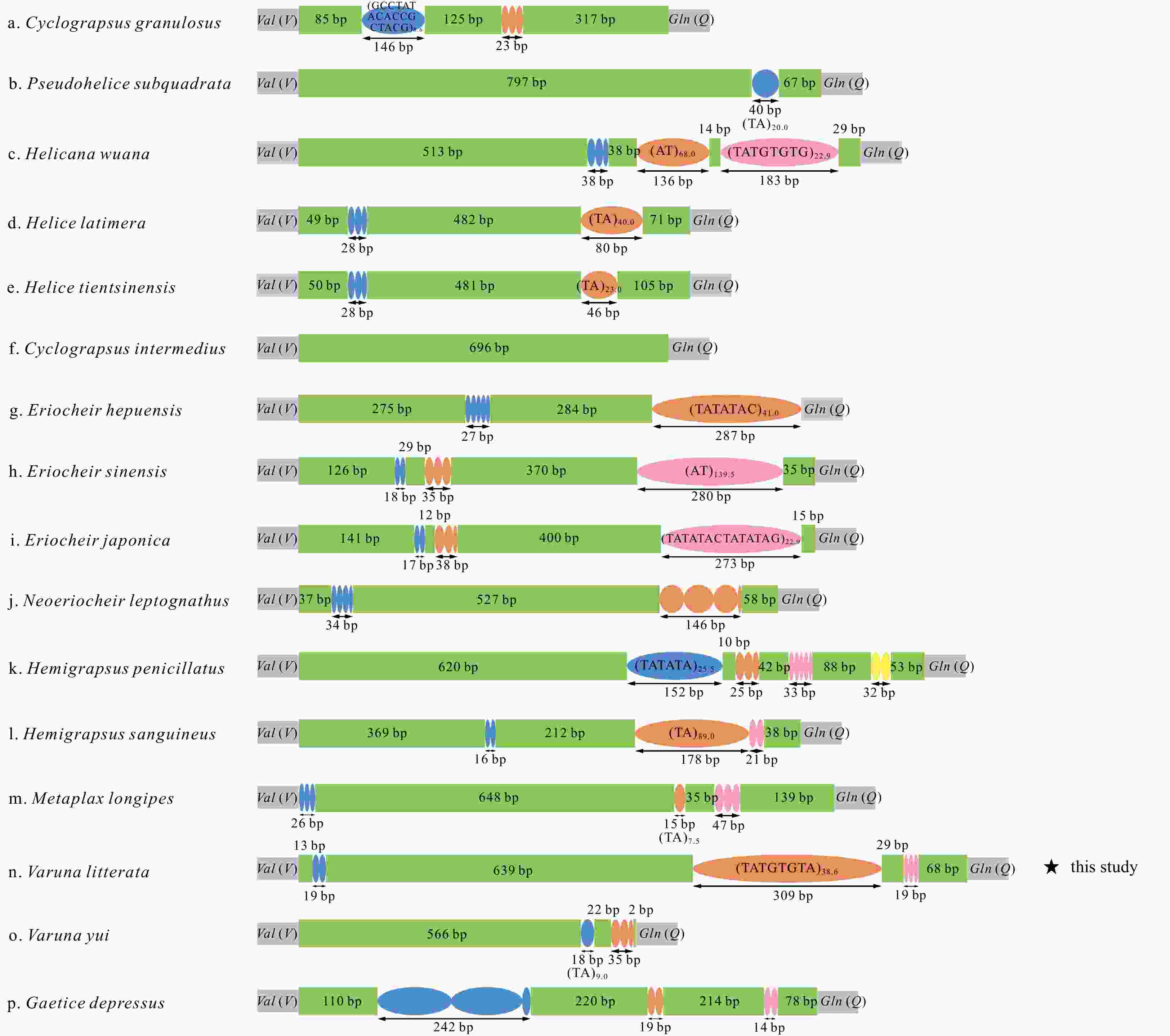
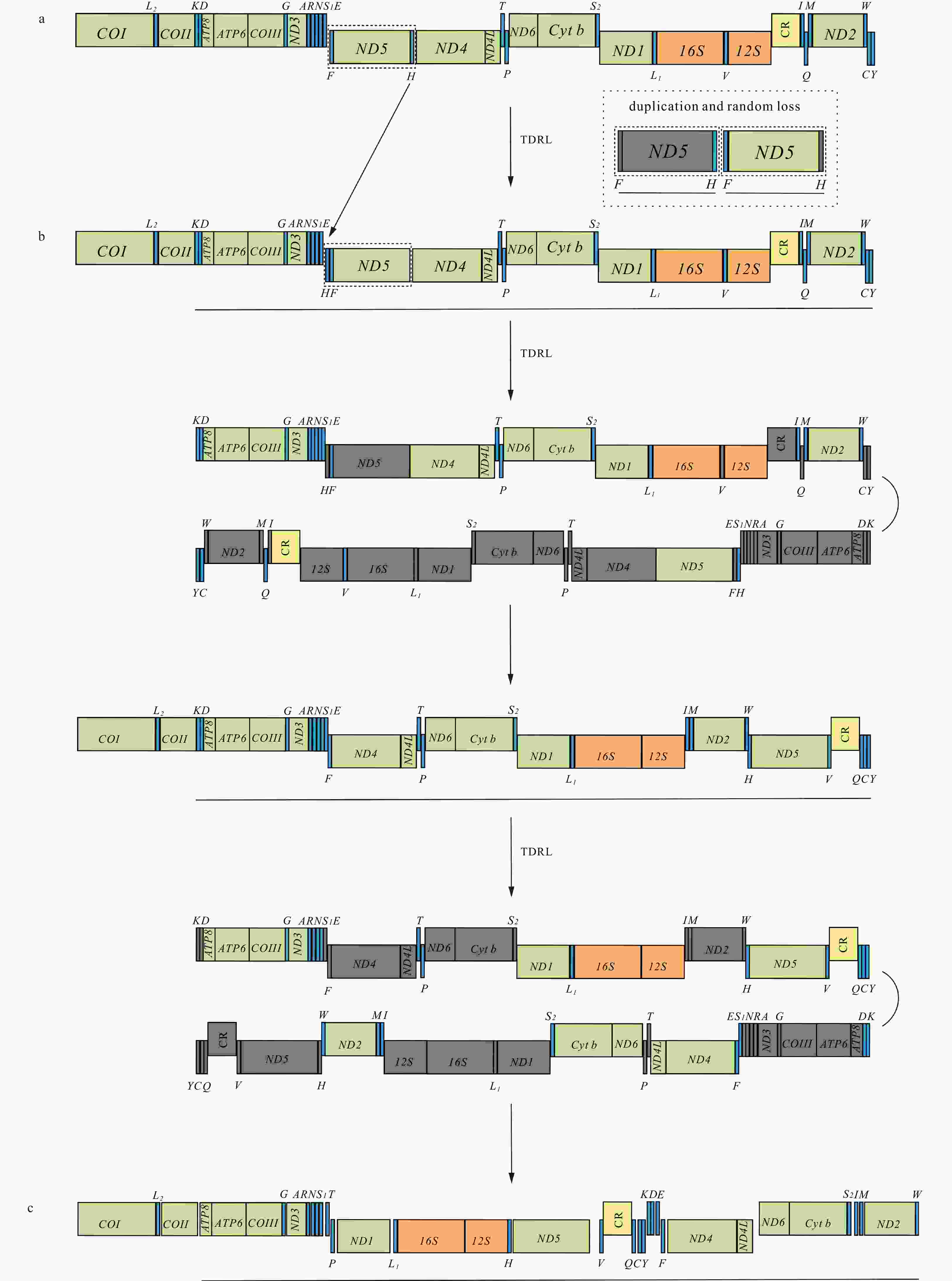
Figure 6. Inferred intermediate steps between the ancestral gene arrangement of crustaceans and Varunidae mitogenomes. a. The ancestral gene arrangement of crustaceans; b. the results of one tandem duplication/random loss (TDRL) event and the ancestral gene arrangement of Brachyura; c. the results of two TDRL events and the final gene arrangement in Varuna litterata and 15 other varunid species. The duplicated gene block is underlined and the lost genes are labeled with gray.
Table 1. Features of the mitochondrial genome of Varuna litterata
Gene Position Length/
bpAmino
acidStart/Stop
codonAnticodon Intergenic
regionStrand From To COI 1 bp 1534 bp 1534 511 ATG/T – 0 H Leu (L2) 1535 bp 1600 bp 66 – – TAA 5 H COII 1606 bp 2310 bp 705 234 ATG/TAA – 12 H ATP8 2323 bp 2484 bp 162 53 ATG/TAA – −7 H ATP6 2478 bp 3152 bp 675 224 ATT/TAA – −1 H COIII 3152 bp 3943 bp 792 263 ATG/TAA – −1 H Gly (G) 3943 bp 4007 bp 65 – – TCC 0 H ND3 4008 bp 4358 bp 351 116 ATT/TAA – 1 H Ala (A) 4360 bp 4422 bp 63 – – TGC 2 H Arg (R) 4425 bp 4487 bp 63 – – TCG −1 H Asn (N) 4487 bp 4551 bp 65 – – GTT 0 H Ser (S1) 4552 bp 4618 bp 67 – – TCT 22 H Thr (T) 4641 bp 4705 bp 65 – – TGT 2 H Pro (P) 4708 bp 4771 bp 64 – – TGG 20 L ND1 4792 bp 5727 bp 936 311 ATG/TAA – 31 L Leu (L1) 5759 bp 5825 bp 67 – – TAG 0 L 16S 5826 bp 7198 bp 1373 – – – 0 L 12S 7199 bp 8087 bp 889 – – – 0 L His (H) 8088 bp 8150 bp 63 – – GTG 6 L ND5 8157 bp 9890 bp 1734 577 ATA/TAA – 126 L Val (V) 10 017 bp 10 089 bp 73 – – TAC 0 L CR 10 090 bp 11 185 bp 1096 – – – 0 H Gln (Q) 11 186 bp 11 254 bp 69 – – TTG 11 L Cys (C) 11 266 bp 11 328 bp 63 – – GCA 0 L Tyr (Y) 11 329 bp 11 393 bp 65 – – GTA 2 L Lys (K) 11 396 bp 11 465 bp 70 – – TTT −2 H Asp (D) 11 464 bp 11 531 bp 68 – – GTC 6 H Glu (E) 11 538 bp 11 602 bp 65 – – TTC 2 H Phe (F) 11 605 bp 11 669 bp 65 – – GAA 14 L ND4 11 684 bp 13 021 bp 1338 445 ATG/TAG – −7 L ND4L 13 015 bp 13 326 bp 312 103 ATA/TAA – 87 L ND6 13 414 bp 13 923 bp 510 169 ATT/TAA – −1 H Cyt b 13 923 bp 15 057 bp 1135 378 ATG/T – 0 H Ser (S2) 15 058 bp 15 124 bp 67 – – TGA 20 H Ile (I) 15 145 bp 15 209 bp 65 – – GAT 4 H Met (M) 15 214 bp 15 281 bp 68 – – CAT 0 H ND2 15 282 bp 16 292 bp 1011 336 ATC/TAG – −2 H Trp (W) 16 291 bp 16 358 bp 68 – – TCA 9 H Note: – represents no data. CR is abbreviation of control region. Table 2. The percentage content of composition and skewness of Varuna litterata mitogenome
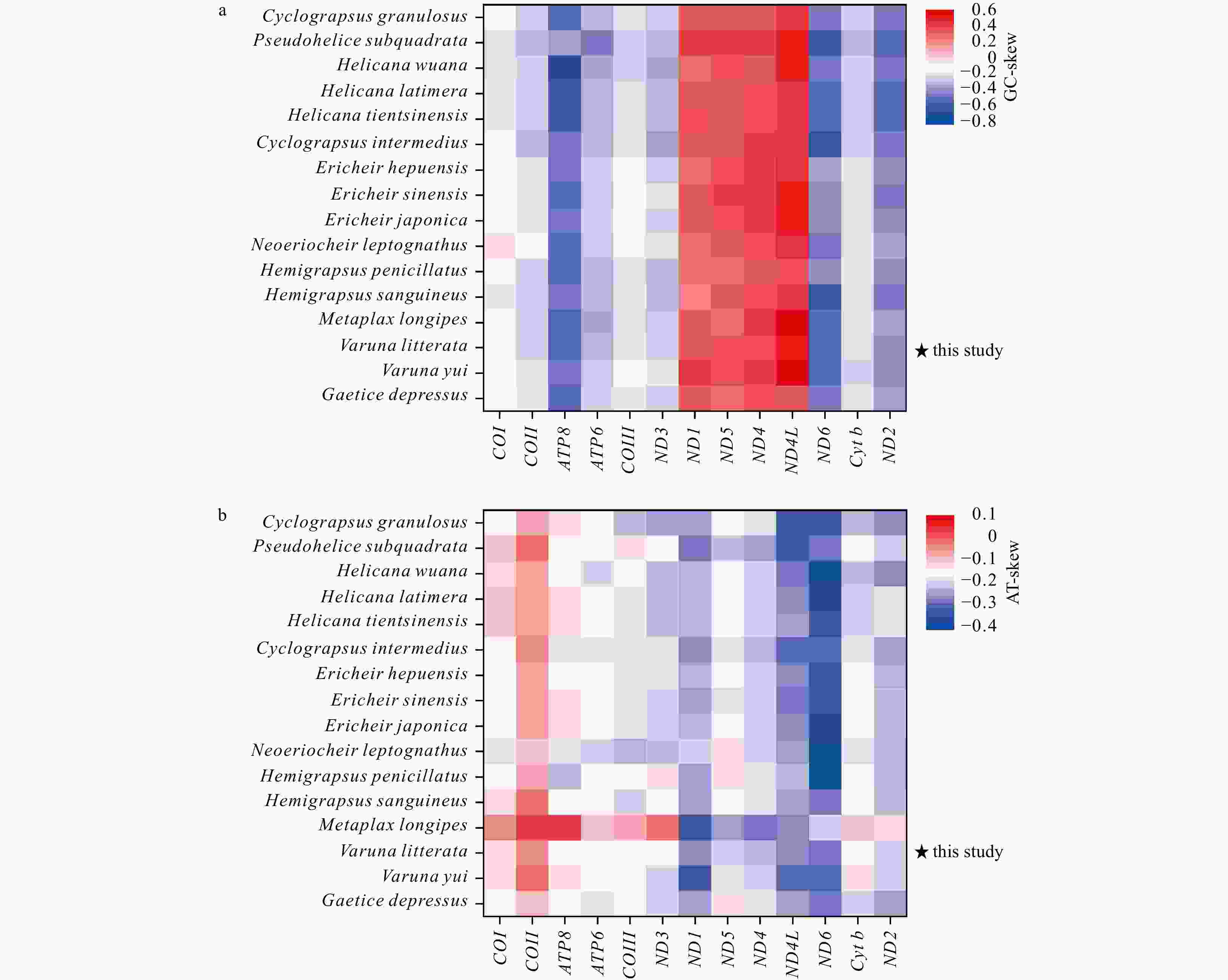
A/% T/% G/% C/% (A+T)/% AT-skew GC-skew Length/bp Mitogenome 35.2 36.2 10.8 17.8 71.4 −0.014 −0.243 16 368 PCGs 28.2 40.3 15.6 16.0 68.4 −0.177 −0.011 11 195 COI 27.8 35.2 16.9 20.1 63.0 −0.118 −0.088 1539 COII 32.5 34.8 12.8 20.0 67.2 −0.034 −0.221 705 ATP8 35.8 48.8 3.7 11.7 84.6 −0.153 −0.520 162 ATP6 28.9 39.0 11.7 20.4 67.9 −0.148 −0.272 675 COIII 26.8 37.0 15.2 21.1 63.8 −0.160 −0.164 792 ND3 30.8 41.6 10.3 17.4 72.4 −0.150 −0.258 351 ND1 25.4 43.5 20.7 10.4 68.9 −0.262 0.333 936 ND5 28.1 40.7 20.9 10.3 68.8 −0.184 0.342 1734 ND4 28.1 42.6 19.7 9.6 70.7 −0.205 0.342 1338 ND4L 27.9 47.4 18.6 6.1 75.3 −0.260 0.506 282 ND6 26.7 47.5 6.9 19.0 74.1 −0.280 −0.470 549 Cyt b 26.9 37.5 14.4 21.2 64.4 −0.166 −0.193 1135 ND2 29.5 43.7 8.1 18.7 73.2 −0.195 −0.395 1011 16S rRNA 40.4 39.0 13.6 7.0 79.4 0.018 0.322 1373 12S rRNA 41.4 38.7 12.7 7.2 80.1 0.034 0.277 889 tRNAs 38.0 35.8 14.9 11.3 73.8 0.031 0.134 1454 CR 38.0 42.2 11.1 8.7 80.2 −0.053 0.124 1096 Table 3. The percentage content of composition and skewness of mitogenome in 16 Varunidae species
Species A/% T/% G/% C/% (A + T)/% AT-skew GC-skew Length/bp Cyclograpsus granulosus 33.1 36.1 11.2 19.5 69.3 −0.043 −0.272 16 300 Pseudohelice subquadrata 34.2 33.5 10.5 21.7 67.7 0.010 −0.347 16 898 Helicana wuana 33.0 35.5 11.5 20.0 68.4 −0.037 −0.269 16 359 Helice latimera 34.0 35.1 11.0 19.9 69.1 −0.017 −0.290 16 246 Helice tientsinensis 33.9 35.1 11.0 19.9 69.1 −0.017 −0.289 16 212 Cyclograpsus intermedius 34.7 35.9 10.7 18.7 70.6 −0.017 −0.270 16 184 Eriocheir hepuensis 35.1 36.4 10.8 17.7 71.5 −0.018 −0.245 16 335 Eriocheir sinensis 35.3 36.4 10.7 17.7 71.6 −0.015 −0.248 16 354 Eriocheir japonica 35.2 36.5 10.7 17.7 71.6 −0.018 −0.245 16 352 Neoeriocheir leptognathus 35.6 39.0 10.1 15.3 74.6 −0.046 −0.206 16 143 Hemigrapsus penicillatus 34.1 36.4 11.4 18.1 70.5 −0.033 −0.229 16 486 Hemigrapsus sanguineus 34.3 35.5 11.2 19.1 69.8 −0.018 −0.260 16 275 Metaplax longipes 37.6 33.8 10.6 17.9 71.4 0.053 −0.257 16 305 Varuna litterata 35.2 36.2 10.8 17.8 71.4 −0.014 −0.243 16 368 Varuna yui 35.7 36.5 10.2 17.6 72.2 −0.011 −0.265 15 915 Gaetice depressus 35.4 37.6 10.5 16.5 73.0 −0.030 −0.223 16 288 -
[1] Alcock A W. 1900. Materials for a carcinological fauna of India. No. 6: The Brachyura Catometopa or Grapsoidea. Journal of the Asiatic Society of Bengal, 69(3): 279–456 [2] Arndt A, Smith M J. 1998. Mitochondrial gene rearrangement in the sea cucumber genus Cucumaria. Molecular Biology and Evolution, 15(8): 1009–1016. doi: 10.1093/oxfordjournals.molbev.a025999 [3] Basso A, Babbucci M, Pauletto M, et al. 2017. The highly rearranged mitochondrial genomes of the crabs Maja crispata and Maja squinado (Majidae) and gene order evolution in Brachyura. Scientific Reports, 7(1): 4096. doi: 10.1038/s41598-017-04168-9 [4] Benson G. 1999. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Research, 27(2): 573–580. doi: 10.1093/nar/27.2.573 [5] Bernt M, Donath A, Jühling F, et al. 2013. MITOS: improved de novo metazoan mitochondrial genome annotation. Molecular Phylogenetics and Evolution, 69(2): 313–319. doi: 10.1016/j.ympev.2012.08.023 [6] Bernt M, Merkle D, Ramsch K, et al. 2007. CREx: inferring genomic rearrangements based on common intervals. Bioinformatics, 23(21): 2957–2958. doi: 10.1093/bioinformatics/btm468 [7] Boore J L. 1999. Animal mitochondrial genomes. Nucleic Acids Research, 27(8): 1767–1780. doi: 10.1093/nar/27.8.1767 [8] Boore J L, Lavrov D V, Brown W M. 1998. Gene translocation links insects and crustaceans. Nature, 392(6677): 667–668. doi: 10.1038/33577 [9] Camargo T R, Wolf M R, Mantelatto F L, et al. 2020. Ultrastructure of spermatozoa of members of Calappidae, Aethridae and Menippidae and discussion of their phylogenetic placement. Acta Zoologica, 101(1): 89–100. doi: 10.1111/azo.12273 [10] Cantatore P, Gadaleta M N, Roberti M, et al. 1987. Duplication and remoulding of tRNA genes during the evolutionary rearrangement of mitochondrial genomes. Nature, 329(6142): 853–855. doi: 10.1038/329853a0 [11] Chen Jianqin, Xing Yuhui, Yao Wenjia, et al. 2018. Characterization of four new mitogenomes from Ocypodoidea & Grapsoidea, and phylomitogenomic insights into thoracotreme evolution. Gene, 675: 27–35. doi: 10.1016/j.gene.2018.06.088 [12] Chen Jianqin, Xing Yuhui, Yao Wenjia, et al. 2019. Phylomitogenomics reconfirm the phylogenetic position of the genus Metaplax inferred from the two grapsid crabs (Decapoda: Brachyura: Grapsoidea). PLoS ONE, 14(1): e0210763. doi: 10.1371/journal.pone.0210763 [13] Dai Aiyun,Yang Siliang. 1991. Crabs of the China Seas. Beijing: China Ocean Press, 473 [14] Davie P J F, Guinot D, Ng P K L. 2015. Systematics and classification of Brachyura. In: Castro P, et al. eds. Treatise on Zoology Antomy, Taxonomy, Biology. Decapoda: The Crustacea. Leiden: Koninklijke Brill NV, 1049–1130 [15] Dierckxsens N, Mardulyn P, Smits G. 2017. NOVOPlasty: de novo assembly of organelle genomes from whole genome data. Nucleic Acids Research, 45(4): e18 [16] Gong Li, Liu Bingjian, Liu Liqin, et al. 2019. The complete mitochondrial genome of Terapon jarbua (Centrarchiformes: Terapontidae) and comparative analysis of the control region among eight Centrarchiformes species. Russian Journal of Marine Biology, 45(2): 137–144. doi: 10.1134/S1063074019020068 [17] Gong Li, Lu Xinting, Wang Zhifu, et al. 2020. Novel gene rearrangement in the mitochondrial genome of Coenobita brevimanus (Anomura: Coenobitidae) and phylogenetic implications for Anomura. Genomics, 112(2): 1804–1812. doi: 10.1016/j.ygeno.2019.10.012 [18] Gyllensten U, Wharton D, Josefsson A, et al. 1991. Paternal inheritance of mitochondrial DNA in mice. Nature, 352(6332): 255–257. doi: 10.1038/352255a0 [19] Jacobs H T, Herbert E R, Rankine J. 1989. Sea urchin egg mitochondrial DNA contains a short displacement loop (D-loop) in the replication origin region. Nucleic Acids Research, 17(22): 8949–8965. doi: 10.1093/nar/17.22.8949 [20] Jamieson B G M, Guinot D, De Forges B R. 1996. Contrasting spermatozoal ultrastructure in two thoracotreme crabs, Cardisoma carnifex (Gecarcinidae) and Varunu litterata (Grapsidae) (Crustacea: Brachyura). Invertebrate Reproduction & Development, 29(2): 111–126 [21] Kalyaanamoorthy S, Minh B Q, Wong T K F, et al. 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods, 14(6): 587–589. doi: 10.1038/nmeth.4285 [22] Katoh K, Misawa K, Kuma K I, et al. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30(14): 3059–3066. doi: 10.1093/nar/gkf436 [23] Kumar S, Stecher G, Li M, et al. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(6): 1547–1549. doi: 10.1093/molbev/msy096 [24] Lavrov D V, Boore J L, Brown W M. 2002. Complete mtDNA sequences of two millipedes suggest a new model for mitochondrial gene rearrangements: duplication and nonrandom loss. Molecular Biology and Evolution, 19(2): 163–169. doi: 10.1093/oxfordjournals.molbev.a004068 [25] Lavrov D V, Brown W M, Boore J L. 2000. A novel type of RNA editing occurs in the mitochondrial tRNAs of the centipede Lithobius forficatus. Proceedings of the National Academy of Sciences of the United States of America, 97(25): 13738–13742. doi: 10.1073/pnas.250402997 [26] Li Ning, Hu Guilin, Hua Baozhen. 2019. Complete mitochondrial genomes of Bittacus strigosus and Panorpa debilis and genomic comparisons of Mecoptera. International journal of biological macromolecules, 140: 672–681. doi: 10.1016/j.ijbiomac.2019.08.152 [27] Li Kui, Liang Aiping. 2018. Hemiptera mitochondrial control region: new sights into the structural organization, phylogenetic utility, and roles of tandem repetitions of the noncoding segment. International Journal of Molecular Sciences, 19(5): 1292. doi: 10.3390/ijms19051292 [28] Li Yuetian, Xin Zhaozhe, Tang Yingyu, et al. 2020. Comparative mitochondrial genome analyses of sesarmid and other brachyuran crabs reveal gene rearrangements and phylogeny. Frontiers in Genetics, 11: 536640. doi: 10.3389/fgene.2020.536640 [29] Lin Fan, Xie Zhuofan, Fazhan H, et al. 2018. The complete mitochondrial genome of Varuna yui (Decapoda: Brachyura: Varunidae) and its phylogeny. Mitochondrial DNA Part B, 3(1): 263–264. doi: 10.1080/23802359.2018.1443043 [30] Liu Yuan, Cui Zhaoxia. 2010. Complete mitochondrial genome of the Asian paddle crab Charybdis japonica (Crustacea: Decapoda: Portunidae): gene rearrangement of the marine brachyurans and phylogenetic considerations of the decapods. Molecular Biology Reports, 37(5): 2559–2569. doi: 10.1007/s11033-009-9773-2 [31] Lowe T M, Chan P P. 2016. tRNAscan-SE On-line: integrating search and context for analysis of transfer RNA genes. Nucleic Acids Research, 44(W1): W54–W57. doi: 10.1093/nar/gkw413 [32] Lu Xinting, Gong Li, Zhang Ying, et al. 2020. The complete mitochondrial genome of Calappa bilineata: the first representative from the family Calappidae and its phylogenetic position within Brachyura. Genomics, 112(3): 2516–2523. doi: 10.1016/j.ygeno.2020.02.003 [33] Lunt D H, Hyman B C. 1997. Animal mitochondrial DNA recombination. Nature, 387(6630): 247. doi: 10.1038/387247a0 [34] Ma Kayan, Qin Jing, Lin Chia-Wei, et al. 2019. Phylogenomic analyses of brachyuran crabs support early divergence of primary freshwater crabs. Molecular Phylogenetics and Evolution, 135: 62–66. doi: 10.1016/j.ympev.2019.02.001 [35] Ma Zhihong, Yang Xuefen, Bercsenyi M, et al. 2015. Comparative mitogenomics of the genus Odontobutis (Perciformes: Gobioidei: Odontobutidae) revealed conserved gene rearrangement and high sequence variations. International Journal of Molecular Sciences, 16(10): 25031–25049. doi: 10.3390/ijms161025031 [36] Martin J W, Davis G E. 2001. An updated classification of the recent Crustacea. In: Heyning J, Harris M J, Brown V B, eds. Natural History Museum of Los Angeles County: Science Series 39, 1–124 [37] Masta S E, Boore J L. 2004. The complete mitochondrial genome sequence of the spider Habronattus oregonensis reveals rearranged and extremely truncated tRNAs. Molecular Biology and Evolution, 21(5): 893–902. doi: 10.1093/molbev/msh096 [38] Moritz C, Brown W M. 1987. Tandem duplications in animal mitochondrial DNAs: variation in incidence and gene content among lizards. Proceedings of the National Academy of Sciences of the United States of America, 84(20): 7183–7187. doi: 10.1073/pnas.84.20.7183 [39] Moritz C, Dowling T E, Brown W M. 1987. Evolution of animal mitochondrial DNA: relevance for population biology and systematics. Annual Review of Ecology and Systematics, 18: 269–292. doi: 10.1146/annurev.es.18.110187.001413 [40] Muse S V. 2000. Examining rates and patterns of nucleotide substitution in plants. Plant Molecular Biology, 42(1): 25–43. doi: 10.1023/A:1006319803002 [41] Ng N K. 2006. The Systematics of the Crabs of the Family Varunidae (Brachyura, Decapoda). Singapore: National University of Singapore [42] Ng P K L, Guinot D, Davie P J F. 2008. Systema brachyurorum: Part I. An annotated checklist of extant brachyuran crabs of the world. The Raffles Bulletin of Zoology, 17: 1–286 [43] Nguyen L T, Schmidt H A, Von Haeseler A, et al. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32(1): 268–274. doi: 10.1093/molbev/msu300 [44] Ojala D, Montoya J, Attardi G. 1981. tRNA punctuation model of RNA processing in human mitochondria. Nature, 290(5806): 470–474. doi: 10.1038/290470a0 [45] Perna N T, Kocher T D. 1995. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. Journal of Molecular Evolution, 41(3): 353–358. doi: 10.1007/BF01215182 [46] Ronquist F, Teslenko M, Van Der Mark P, et al. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61(3): 539–542. doi: 10.1093/sysbio/sys029 [47] Rozas J, Ferrer-Mata A, Sánchez-DelBarrio J C, et al. 2017. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Molecular Biology and Evolution, 34(12): 3299–3302. doi: 10.1093/molbev/msx248 [48] Sanchez G, Tomano S, Yamashiro C, et al. 2016. Population genetics of the jumbo squid Dosidicus gigas (Cephalopoda: Ommastrephidae) in the northern Humboldt Current system based on mitochondrial and microsatellite DNA markers. Fisheries Research, 175: 1–9. doi: 10.1016/j.fishres.2015.11.005 [49] Sato M, Sato K. 2013. Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochimica et Biophysica Acta-Molecular Cell Research, 1833(8): 1979–1984. doi: 10.1016/j.bbamcr.2013.03.010 [50] Schubart C D, Cuesta J A, Diesel R, et al. 2000. Molecular phylogeny, taxonomy, and evolution of nonmarine lineages within the American grapsoid crabs (Crustacea: Brachyura). Molecular Phylogenetics and Evolution, 15(2): 179–190. doi: 10.1006/mpev.1999.0754 [51] Schubart C D, Cuesta J A, Felder D L. 2002. Glyptograpsidae, a new brachyuran family from Central America: larval and adult morphology, and a molecular phylogeny of the Grapsoidea. Journal of Crustacean Biology, 22(1): 28–44. doi: 10.1163/20021975-99990206 [52] Stothard P, Wishart D S. 2005. Circular genome visualization and exploration using CGView. Bioinformatics, 21(4): 537–539. doi: 10.1093/bioinformatics/bti054 [53] Talavera G, Castresana J. 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56(4): 564–577. doi: 10.1080/10635150701472164 [54] Tan Munhua, Gan Hanming, Lee Yinpeng, et al. 2018. ORDER within the chaos: Insights into phylogenetic relationships within the Anomura (Crustacea: Decapoda) from mitochondrial sequences and gene order rearrangements. Molecular Phylogenetics and Evolution, 127: 320–331. doi: 10.1016/j.ympev.2018.05.015 [55] Tan Munhua, Gan Hanming, Lee Yinpeng, et al. 2019. Comparative mitogenomics of the Decapoda reveals evolutionary heterogeneity in architecture and composition. Scientific Reports, 9(1): 10756. doi: 10.1038/s41598-019-47145-0 [56] Tang Boping, Liu Yu, Xin Zhaozhe, et al. 2018. Characterisation of the complete mitochondrial genome of Helice wuana (Grapsoidea: Varunidae) and comparison with other Brachyuran crabs. Genomics, 110(4): 221–230. doi: 10.1016/j.ygeno.2017.10.001 [57] Tu Chin-Hung. 1992. Studies on the larval culture of Varuna litterata [dissertation]. Kaohsiung, China: National Sun Yat-Sen University [58] Wang Xiaoyan, Huang Yuan, Liu Nian, et al. 2015. Seven complete mitochondrial genome sequences of bushtits (Passeriformes, Aegithalidae, Aegithalos): the evolution pattern in duplicated control regions. Mitochondrial DNA, 26(3): 350–356. doi: 10.3109/19401736.2014.1003821 [59] Wang Zhengfei, Shi Xuejia, Tao Yitao, et al. 2019. The complete mitochondrial genome of Parasesarma pictum (Brachyura: Grapsoidea: Sesarmidae) and comparison with other Brachyuran crabs. Genomics, 111(4): 799–807. doi: 10.1016/j.ygeno.2018.05.002 [60] Wang Qi, Tang Dan, Guo Huayun, et al. 2020. Comparative mitochondrial genomic analysis of Macrophthalmus pacificus and insights into the phylogeny of the Ocypodoidea & Grapsoidea. Genomics, 112(1): 82–91. doi: 10.1016/j.ygeno.2019.12.012 [61] Xin Zhaozhe, Liu Yu, Zhang Daizhen, et al. 2017. Mitochondrial genome of Helice tientsinensis (Brachyura: Grapsoidea: Varunidae): gene rearrangements and higher-level phylogeny of the Brachyura. Gene, 627: 307–314. doi: 10.1016/j.gene.2017.06.036 [62] Zhang Dong, Gao Fangluan, Jakovlić I, et al. 2020a. PhyloSuite: an integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20(1): 348–355. doi: 10.1111/1755-0998.13096 [63] Zhang Bo, Wu Yingying, Wang Xin, et al. 2020b. Comparative analysis of mitochondrial genome of a deep-sea crab Chaceon granulates reveals positive selection and novel genetic features. Journal of Oceanology and Limnology, 38(2): 427–437. doi: 10.1007/s00343-019-8364-x [64] Zhuang Xuan, Cheng C H C. 2010. ND6 gene “lost” and found: evolution of mitochondrial gene rearrangement in Antarctic notothenioids. Molecular Biology and Evolution, 27(6): 1391–1403. doi: 10.1093/molbev/msq026 -
 Zhang Ying Supplemental Material.pdf
Zhang Ying Supplemental Material.pdf
-




 下载:
下载: