The behavioral and antioxidant response of the bivalve Gomphina veneriformis to sediment burial effect
-
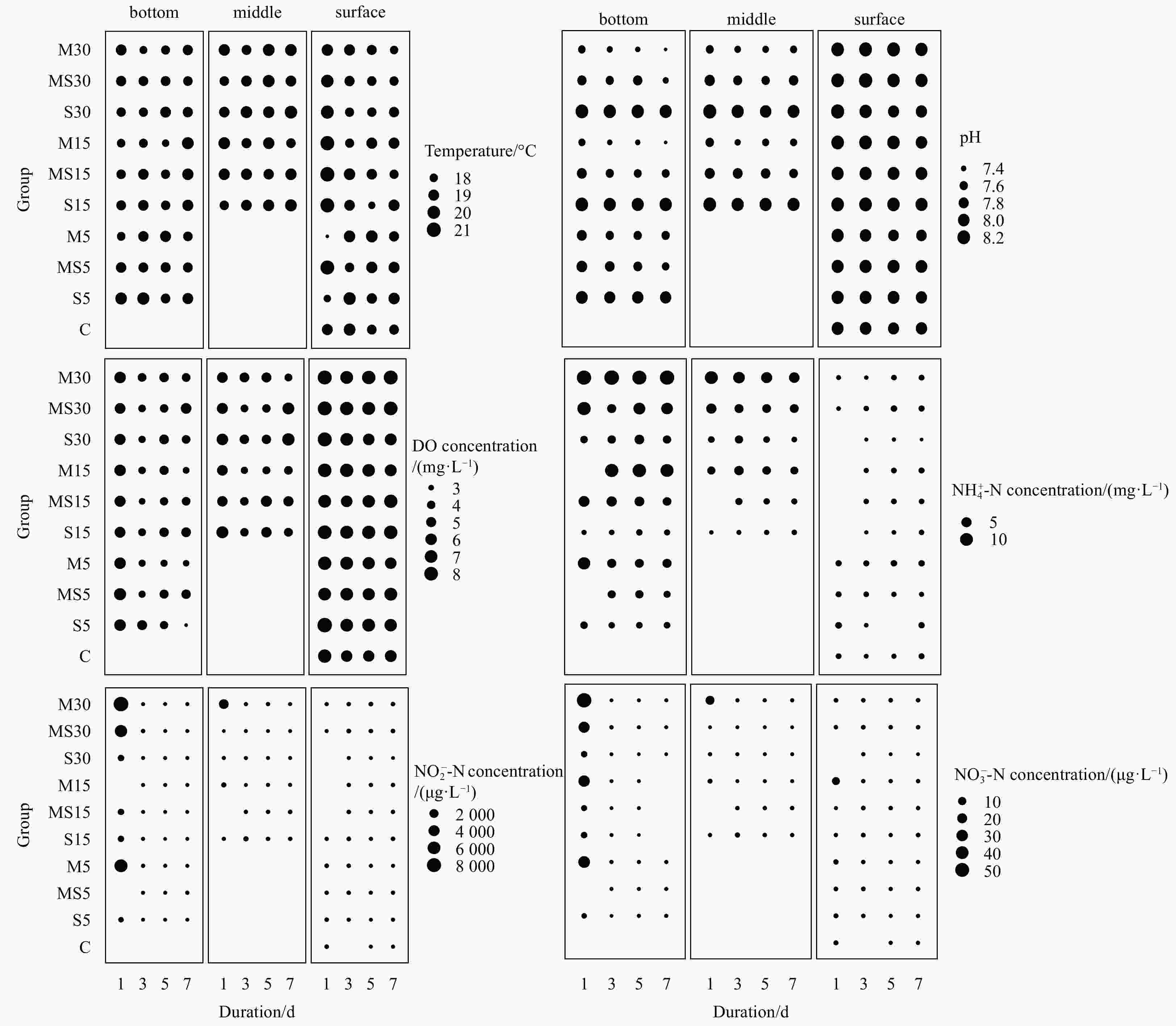
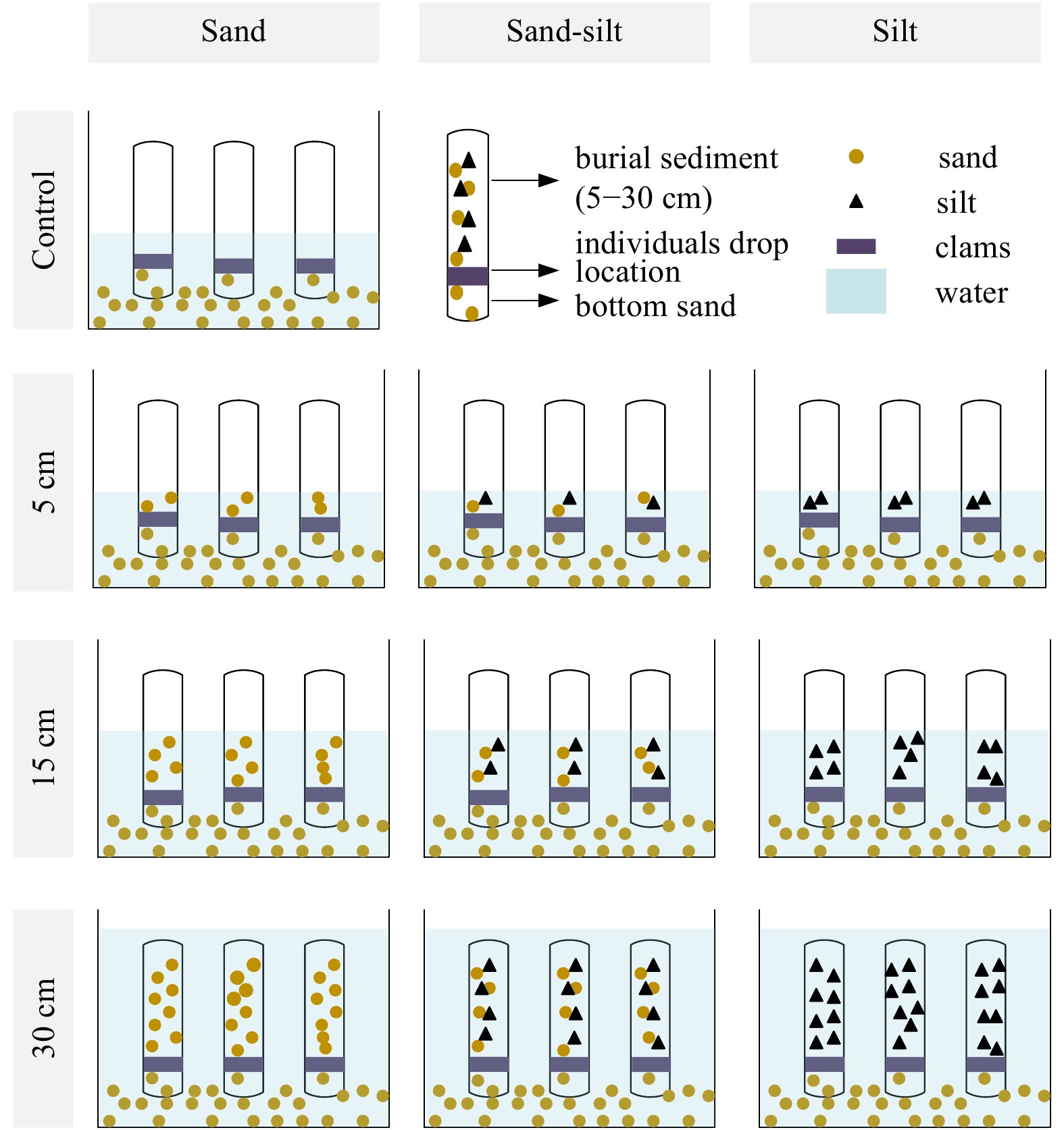
Abstract: A laboratory-based microcosm experiment was carried out to examine both the behavioral and antioxidant response of the clam Gomphina veneriformis under the conditions of 3 types of burial material (sand, silt, silt-sand mixture) with 3 burial depths (5 cm, 15 cm, 30 cm). The concentration of dissolved oxygen decreased significantly after 3 d of burial in all experimental groups. In silt and sand-silt mixture groups, the interstitial water quality became worsened with lower pH, and higher
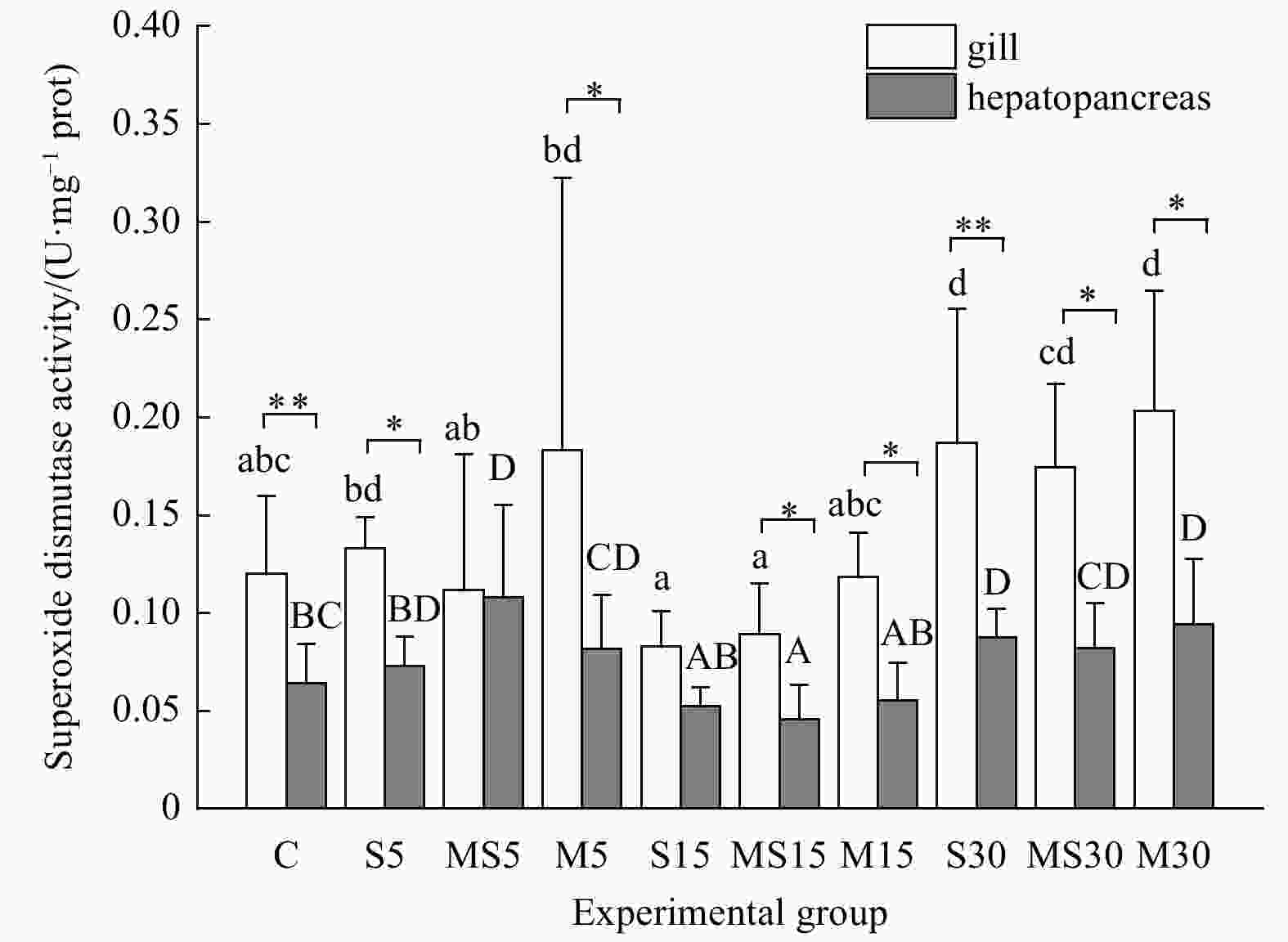
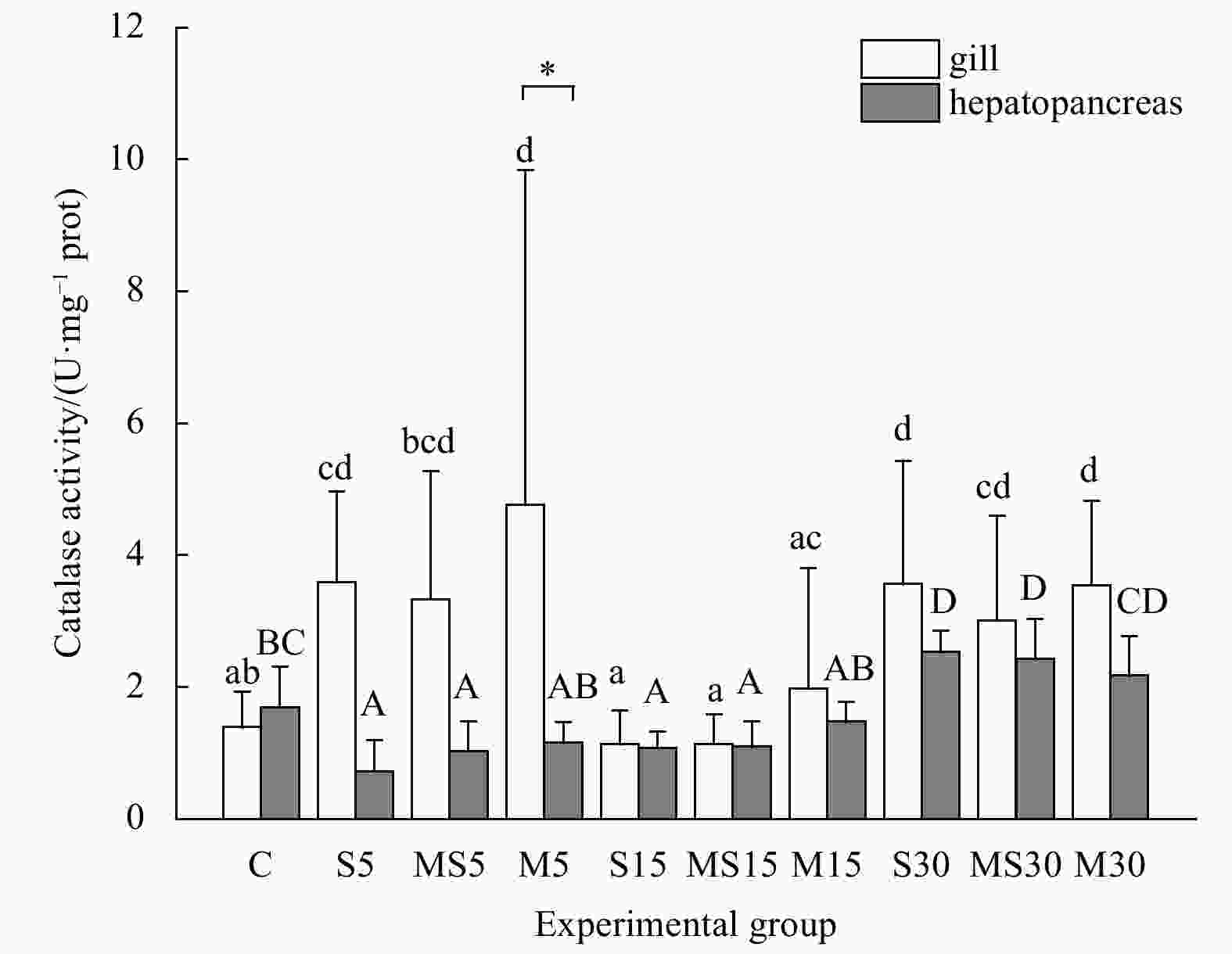
$ {\rm {NH}}_4^+$ -N concentration, where clam mortality occurred simultaneously. However, clam samples in all sand groups and 5 cm, 15 cm sand-silt mixture groups survived well for 8 d. Obviously fewer individuals left in the bottom sand in the 15 cm, 30 cm silt groups and 30 cm sand-silt mixture groups than in the 5 cm groups. Therefore, it suggests that adding silt and increasing burial depth could stimulate the vertical movement of organisms and cause lethal effects. It was found that the burial depth was the key factor that influenced the activities of antioxidant enzymes, such as superoxide dismutase (SOD) and catalase (CAT). The SOD and CAT activities in the gills and hepatopancreases of organisms both showed significant up-regulation in 30 cm burial depth after buried for 8 d. Higher enzyme activities were found in gills than in hepatopancreases, which indicated that the gills of the bivalve G. veneriformis were more susceptible to burial effects than hepatopancreases. Overall, this study shows that sediment burial could cause effects on the biological behavior and antioxidant enzyme activities. -
Figure 3. The superoxide dismutase (SOD) activities in gills and hepatopancreases of G. veneriformis in each experimental group. Error bars indicate standard deviation (n>5). The bars marked with different letters indicates there are significant differences among experimental groups, same letters no difference. Lower-case letters indicate difference analysis of SOD activities among groups in gills and capital letters in hepatopancreases. Asterisks indicated different SOD activities between gills and hepatopancreas in the same group. *p<0.05; **p<0.01.
Figure 4. The catalase (CAT) activities in gills and hepatopancreases of G. veneriformis in each experimental group. Error bars indicate the standard deviation (n>3). The bars marked with different letters indicates there are significant differences among experimental groups, same letters no difference. Lower-case letters indicate difference analysis of CAT activities among groups in gills and capital letters in hepatopancreases. Asterisks indicated different CAT activities between gills and hepatopancreas in the same group. *p<0.05; **p<0.01.
Table 1. The average length and width of clam individuals distributed at different layers in 30 cm burial groups
Layer S30 MS30 M30 Length/cm Width/cm Length/cm Width/cm Length/cm Width/cm 25–30 cm – – 26.35 19.12 26.31 19.36 20–25 cm 29.33 20.04 – – 25.82 18.80 15–20 cm – – 27.39 20.45 27.73 20.28 10–15 cm 24.44 17.56 27.02 19.87 25.74 18.66 5–10 cm 26.59 19.31 25.66 18.96 30.64 22.81 0–5 cm 28.16 20.95 28.42 20.89 25.64 18.78 –5 cm to 0 cm 26.95 19.89 24.81 18.15 25.10 18.51 Note: – means no data. Table 2. The average number (n=3) of individuals distributed at different layers when buried for 8 d
Layer Experimental group C S5 MS5 M5 S15 MS15 M15 S30 MS30 M30 25–30 cm – – – – – – – – 2.00 1.33 20–25 cm – – – – – – – 0.67 – 1.00 15–20 cm – – – – – – – – 1.67 1.00 10–15 cm – – – – 3.00 5.33 5.67 0.33 1.33 1.00 5–10 cm – – – – 0.67 1.33 1.67 2.00 0.67 1.67 0–5 cm – 4.33 4.00 2.00 2.33 0.67 2.00 2.33 3.33 2.67 –5–0 cm 10.00 5.67 6.00 8.00 4.00 2.67 0.67 4.33 1.00 1.33 Note: – means no data. -
[1] Basha P S, Rani A U. 2003. Cadmium-induced antioxidant defense mechanism in freshwater teleost Oreochromis mossambicus (Tilapia). Ecotoxicology and Environmental Safety, 56(2): 218–221. doi: 10.1016/S0147-6513(03)00028-9 [2] Bellchambers L, Richardson A. 1995. The effect of substrate disturbance and burial depth on the venerid clam, Katelysia scalarina (Lamarck, 1818). Journal of Shellfish Research, 14(1): 41–44 [3] Bijkerk R. 1988. Ontsnappen of begraven blijven: de effecten op bodemdieren van een verhoogde sedimentatie als gevolg van baggerwerkzaamheden: literatuuronderzoek. Groningen: RDD Aquatic Ecosystems, 1–72 [4] Bolam S G. 2011. Burial survival of benthic macrofauna following deposition of simulated dredged material. Environmental Monitoring and Assessment, 181(1–4): 13–27 [5] Bradford M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72: 248–254 [6] Chandrasekara W U, Frid C L J. 1998. A laboratory assessment of the survival and vertical movement of two epibenthic gastropod species, Hydrobia ulvae (Pennant) and Littorina littorea (Linnaeus), after burial in sediment. Journal of Experimental Marine Biology and Ecology, 221(2): 191–207. doi: 10.1016/S0022-0981(97)00123-8 [7] Chen Rong, Zheng Weiyun, Yu Qun, et al. 2002. Effect of oil pollution on antioxidant enzyme of oyster (Ostrea cucullata). Acta Scientiae Circumstantiae (in Chinese), 22(3): 385–388 [8] Cong Ming, Wu Huifeng, Liu Xiaoli, et al. 2012. Effects of heavy metals on the expression of a zinc-inducible metallothionein-III gene and antioxidant enzyme activities in Crassostrea gigas. Ecotoxicology, 21(7): 1928–1936. doi: 10.1007/s10646-012-0926-z [9] Conroy E, Turner J N, Rymszewicz A, et al. 2017. Further insights into the responses of macroinvertebrate species to burial by sediment. Hydrobiologia, 805(1): 399–411 [10] Greco L, Pellerin J, Capri E, et al. 2011. Physiological effects of temperature and a herbicide mixture on the soft-shell clam Mya arenaria (Mollusca, Bivalvia). Environmental Toxicology and Chemistry, 30(1): 132–141. doi: 10.1002/etc.359 [11] Jackson M J, James R. 1979. The influence of bait digging on cockle, Cerastoderma edule, populations in North Norfolk. Journal of Applied Ecology, 16(3): 671–679. doi: 10.2307/2402844 [12] Ji Xiao. 2014. Burial effects of dredged material on Ruditapes philippinarum and Perinereis aibuhitensis (in Chinese) [dissertation]. Shanghai: Shanghai Ocean University [13] Kranz P M. 1974. The anastrophic burial of bivalves and its paleoecological significance. The Journal of Geology, 82(2): 237–265. doi: 10.1086/627961 [14] Maurer D, Keck R T, Tinsman J C, et al. 1981a. Vertical migration and mortality of benthos in dredged material: Part I. Mollusca. Marine Environmental Research, 4(4): 299–319. doi: 10.1016/0141-1136(81)90043-X [15] Maurer D, Keck R T, Tinsman J C, et al. 1981b. Vertical migration and mortality of benthos in dredged material: Part II. Crustacea. Marine Environmental Research, 5(4): 301–317. doi: 10.1016/0141-1136(81)90014-3 [16] Maurer D, Keck R T, Tinsman J C, et al. 1982. Vertical migration and mortality of benthos in dredged material: Part III. Polychaeta. Marine Environmental Research, 6(1): 49–68. doi: 10.1016/0141-1136(82)90007-1 [17] Maurer D, Keck R T, Tinsman J C, et al. 1986. Vertical migration and mortality of marine benthos in dredged material: a synthesis. Internationale Revue der gesamten Hydrobiologie und Hydrographie, 71(1): 49–63. doi: 10.1002/iroh.19860710106 [18] Naser H A. 2011. Effects of reclamation on macrobenthic assemblages in the coastline of the Arabian Gulf: a microcosm experimental approach. Marine Pollution Bulletin, 62(3): 520–524. doi: 10.1016/j.marpolbul.2010.11.032 [19] Nunes B, Nunes J, Soares A M V M, et al. 2017. Toxicological effects of paracetamol on the clam Ruditapes philippinarum: exposure vs recovery. Aquatic Toxicology, 192: 198–206. doi: 10.1016/j.aquatox.2017.09.015 [20] Park K, Kim R, Park J J, et al. 2012. Ecotoxicological evaluation of tributyltin toxicity to the equilateral venus clam, Gomphina veneriformis (Bivalvia: Veneridae). Fish and Shellfish Immunology, 32(3): 426–433. doi: 10.1016/j.fsi.2011.11.031 [21] Powilleit M, Graf G, Kleine J, et al. 2009. Experiments on the survival of six brackish macro-invertebrates from the Baltic Sea after dredged spoil coverage and its implications for the field. Journal of Marine Systems, 75(3–4): 441–451 [22] Ross S W, Dalton D A, Kramer S, et al. 2001. Physiological (antioxidant) responses of estuarine fishes to variability in dissolved oxygen. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 130(3): 289–303 [23] Séguin A, Harvey É, Archambault P, et al. 2014. Body size as a predictor of species loss effect on ecosystem functioning. Scientific Reports, 4: 4616 [24] Stanley S M. 1970. Relation of Shell Form to Life Habits of the Bivalvia (Mollusca). Boulder, Colorado: Geological Society of America, 45–85 [25] Stebbing A R D. 1982. Hormesis—the stimulation of growth by low levels of inhibitors. Science of the Total Environment, 22(3): 213–234. doi: 10.1016/0048-9697(82)90066-3 [26] Suzuki J, Imamura M, Nakano D, et al. 2018. Effects of water turbidity and different temperatures on oxidative stress in caddisfly (Stenopsyche marmorata) larvae. Science of the Total Environment, 630: 1078–1085. doi: 10.1016/j.scitotenv.2018.02.286 [27] Trueman E R. 1983. Locomotion in molluscs. In: Saleuddin A S M, Wilbur K M, eds. The Mollusca. New York: Academic Press, 155–198 [28] Woo S, Denis V, Won H, et al. 2013. Expressions of oxidative stress-related genes and antioxidant enzyme activities in Mytilus galloprovincialis (Bivalvia, Mollusca) exposed to hypoxia. Zoological Studies, 52(1): 15. doi: 10.1186/1810-522X-52-15 [29] Woodward G, Ebenman B, Emmerson M, et al. 2005. Body size in ecological networks. Trends in Ecology & Evolution, 20(7): 402–409 [30] Wu Huifeng, Liu Xiaoli, Zhao Jianmin, et al. 2012. Regulation of metabolites, gene expression, and antioxidant enzymes to environmentally relevant lead and zinc in the halophyte Suaeda salsa. Journal of Plant Growth Regulation, 32(2): 353–361 [31] Yanez B, Carballo J L, Olabrarria C, et al. 2008. Recovery of macrobenthic assemblages following experimental sand burial. Oceanologia, 50(3): 391–420 [32] Yang Guojun, Song Lun, Lu Xiaoqian, et al. 2017. Effect of the exposure to suspended solids on the enzymatic activity in the bivalve Sinonovacula constricta. Aquaculture and Fisheries, 2(1): 10–17. doi: 10.1016/j.aaf.2017.01.001 [33] You Zhongjie, Wang Yinong, Yan Zhengrong, et al. 1992. A study on the living habits of the sand clam Gomphina veneriformis (Lamarck). Donghai Marine Science (in Chinese), 10(3): 70–76 -






 下载:
下载: